本翻譯僅作學術交流用,無商業意圖,請勿轉載,如有疑議問請來信
在最新研究中發現,肥胖人群的內臟脂肪通過門靜脈向體內釋放發炎蛋白質(如白介素6),這可能是造成系統性炎症和胰島素抗性的關鍵因素。此研究提供了一個機制鏈接,解釋了腹部肥胖如何影響整體健康,增加了2型糖尿病和心血管疾病的風險。
Abstact
Although excess visceral fat is associated with noninfectious inflammation, it is not clear whether visceral fat is simply associated with or actually causes metabolic disease in humans. To evaluate the hypothesis that visceral fat promotes systemic inflammation by secreting inflammatory adipokines into the portal circulation that drains visceral fat, we determined adipokine arteriovenous concentration differences across visceral fat, by obtaining portal vein and radial artery blood samples, in 25 extremely obese subjects (mean ± SD BMI 54.7 ± 12.6 kg/m2) during gastric bypass surgery at Barnes-Jewish Hospital in St. Louis, Missouri. Mean plasma interleukin (IL)-6 concentration was ∼50% greater in the portal vein than in the radial artery in obese subjects (P = 0.007). Portal vein IL-6 concentration correlated directly with systemic C-reactive protein concentrations (r = 0.544, P = 0.005). Mean plasma leptin concentration was ∼20% lower in the portal vein than in the radial artery in obese subjects (P = 0.0002). Plasma tumor necrosis factor-α, resistin, macrophage chemoattractant protein-1, and adiponectin concentrations were similar in the portal vein and radial artery in obese subjects. These data suggest that visceral fat is an important site for IL-6 secretion and provide a potential mechanistic link between visceral fat and systemic inflammation in people with abdominal obesity.
Excessive visceral fat (i.e., mesenteric and omental fat) is associated with insulin resistance and diabetes (1,2). Accordingly, waist circumference, which correlates with visceral fat mass (3), has been recommended as a clinical marker to identify patients at increased risk for metabolic diseases (4), and large waist circumference is one of the criteria used to diagnose the metabolic syndrome (5). However, the mechanism(s) responsible for the relationship between visceral fat and metabolic abnormalities is not known, and it is not clear whether visceral fat is simply associated with or actually causes metabolic disease.
It has been hypothesized that large amounts of visceral fat cause insulin resistance because lipolysis of visceral adipose tissue triglycerides releases free fatty acids (FFA) directly into the portal vein, which are then transported to the liver (2). Increased delivery of FFA to the liver impairs insulin’s ability to suppress hepatic glucose production, and increased systemic FFA concentration inhibits insulin-mediated glucose disposal in skeletal muscle (6). However, data from studies that examined FFA kinetics in human subjects suggest it is unlikely that lipolytic activity in visceral fat is a major contributor to insulin resistance (7). On average, 20% of portal vein FFA and 14% of total FFA that appear in the systemic circulation are derived from lipolysis of visceral fat in obese subjects (7,8). Therefore, fatty acids released from visceral fat represent only a small percentage of total FFA delivered to liver and muscle tissues.
Visceral fat could cause metabolic abnormalities by secreting inflammatory adipokines, such as interleukin (IL)-6, tumor necrosis factor-α (TNF-α), macrophage chemoattractant protein-1 (MCP-1), and resistin, which induce insulin resistance and diabetes (9,10). In contrast, visceral fat might have beneficial metabolic effects by producing adiponectin (11), which increases insulin sensitivity and decreases glucose intolerance and diabetes (12). However, the importance of adipokine production by visceral fat in the pathogenesis of the metabolic abnormalities associated with obesity has not been carefully studied.
The purpose of the present study was to evaluate the relative contribution of inflammatory adipokines (IL-6, TNF-α, MCP-1, resistin, and leptin) and adiponectin secretion from visceral fat in insulin-resistant subjects with abdominal obesity. Portal vein and peripheral artery plasma concentrations of adipokines were determined in insulin-resistant, extremely obese subjects who had large amounts of visceral fat. We hypothesized that the concentration of inflammatory adipokines would be greater in the portal vein than in the peripheral artery in obese subjects.
摘要
儘管過量的內臟脂肪與非感染性炎症有關,但目前尚不清楚內臟脂肪是僅與代謝疾病相關,還是實際上會導致人類代謝疾病。為了評估內臟脂肪通過分泌炎症性脂肪因子到瀉入內臟脂肪的門靜脈,從而促進全身炎症的假設,我們在密蘇里州聖路易市的巴恩斯-猶太醫院進行胃旁路手術時,採集了25名極度肥胖患者(平均體質指數54.7 ± 12.6公斤/平方米)的門靜脈和橈動脈血樣,確定了脂肪因子的動靜脈濃度差異。平均血漿白介素(IL)-6濃度在門靜脈中比橈動脈高出約50%(P = 0.007)。門靜脈IL-6濃度與全身C反應蛋白濃度直接相關(r = 0.544,P = 0.005)。平均血漿瘦素濃度在門靜脈中比橈動脈低約20%(P = 0.0002)。血漿腫瘤壞死因子-α、抵抗素、單核細胞趨化蛋白-1及脂聯素濃度在肥胖患者的門靜脈與橈動脈中相似。這些數據表明,內臟脂肪是IL-6分泌的重要部位,並為內臟脂肪與腹部肥胖人群的全身炎症之間提供了潛在的機制連接。
過量的內臟脂肪(即腸系膜和大網膜脂肪)與胰島素抗性和糖尿病有關。因此,腰圍,與內臟脂肪質量相關,已被推薦作為臨床標記,用以識別有代謝疾病風險增加的患者,並且大腰圍是診斷代謝症候群的標準之一。然而,內臟脂肪與代謝異常之間的關係負責的機制尚未明了,目前還不清楚內臟脂肪是僅與代謝疾病相關還是實際上會引起代謝疾病。
假設大量的內臟脂肪因內臟脂肪三酸甘油酯的脂解作用釋放自由脂肪酸直接進入門靜脈,然後運輸到肝臟,導致胰島素抗性。肝臟FFA增加會損害胰島素抑制肝葡萄糖產生的能力,並且體系統FFA濃度的增加會抑制胰島素在骨骼肌中介導的葡萄糖利用。然而,研究人類受試者FFA動力學的數據表明,內臟脂肪的脂解活性不太可能是胰島素抗性的主要貢獻因素。平均而言,來自肥胖受試者內臟脂肪脂解的FFA占門靜脈FFA的20%,占全身循環FFA的14%。因此,從內臟脂肪釋放的脂肪酸只是運送到肝臟和肌肉組織的FFA的一小部分。
內臟脂肪可能通過分泌炎症性脂肪因子,如白介素(IL)-6、腫瘤壞死因子-α(TNF-α)、單核細胞趨化蛋白-1(MCP-1)和抵抗素等,引起代謝異常,這些脂肪因子會誘導胰島素抗性和糖尿病。相比之下,內臟脂肪可能通過產生脂聯素具有有益的代謝作用,脂聯素可以增加胰島素敏感性,減少葡萄糖不耐受和糖尿病。然而,內臟脂肪產生的脂肪因子在與肥胖相關的代謝異常的發病機制中的重要性尚未得到仔細研究。
本研究的目的是評估炎症性脂肪因子(IL-6、TNF-α、MCP-1、抵抗素和瘦素)與脂聯素從內臟脂肪中分泌在胰島素抗性患者中的相對貢獻。在擁有大量內臟脂肪的胰島素抗性、極度肥胖的受試者中,確定了門靜脈和周圍動脈血漿中脂肪因子的濃度。我们假设在肥胖受試者中,門靜脈中的炎症性脂肪因子濃度會高於周圍動脈。
研究設計與方法
本研究共有25名III型上半身肥胖的受試者參與(6男19女,BMI 54.7 ± 12.6公斤/平方米,腰圍150 ± 10厘米,年齡42 ± 9歲),他們預定接受開放式胃旁路手術。受試者完成了全面的醫學評估,包括病史、體格檢查、心電圖以及標準的血液和尿液檢測。所有肥胖受試者根據2型糖尿病的病史或高的恆定模型評估分數證實有胰島素抵抗(13)。所有參與這項研究的女性均處於絕經前期。有6名肥胖受試者患有2型糖尿病,正在接受胰島素和二甲雙胍治療;沒有人正在接受噻唑烷二酮類藥物治療。每位受試者在入組前均已簽署書面知情同意書,本研究已獲得密蘇里州聖路易市華盛頓大學醫學院人體研究委員會的批准。
開放式胃旁路手術及上消化道手術於受試者禁食一夜後的早晨在巴恩斯-猶太醫院進行。在手術過程中,於胃縫合或腸切除前從橈動脈和門靜脈同時採集血樣。血樣立即轉移到無菌玻璃EDTA管中(BD Vacutainer;BD Biosciences,英國牛津),置於冰上,並在4°C下以2200g離心10分鐘。然後將血漿樣本放入無菌冷凍瓶中,液氮快速凍結,並存儲於-80°C,待後續分析時使用。
樣本分析
使用商業放射免疫分析套件測量血漿總脂聯素濃度(Linco Research,聖路易斯,MO),並使用商業ELISA套件測量IL-6、TNF-α、抵抗素、MCP-1(Quantakine高敏感;R&D Systems,明尼阿波利斯,MN)以及血漿C反應蛋白(CRP)(ALPCO Diagnostics,溫德姆,NH)濃度。通過速度沉降和定量西方墨點法分析確定血漿高分子量(HMW)和低分子量(LMW)脂聯素(14)。血漿胰島素和瘦素濃度由放射免疫分析測量(Linco Research,聖路易斯,MO)。
統計分析
血樣採集地點間差異的統計顯著性,對於正常分佈且標準差大致相等的數據,使用學生配對t檢驗進行評估。對於非正常分佈或標準差不等的群體間差異的統計顯著性,使用Wilcoxon兩樣本檢驗評估變量。使用皮爾森相關性分析來評估連續變量之間的關聯。當數據非正常分佈時,相關性數據進行對數轉換。P值小於0.05被視為具有統計學意義。所有數據均使用SPSS for Windows軟件,版本12.0(SPSS,芝加哥,IL)進行分析。所有值均以平均值±標準差表示。
結果
在開放式胃旁路手術過程中,從25名極端肥胖的受試者(BMI 54.7 ± 12.6 公斤/平方米,大量內臟脂肪,腰圍 150 ± 10 厘米)同時獲得了門靜脈和周邊動脈的血樣。所有肥胖受試者根據胰島素抵抗的恆定模型評估(13)均顯示有胰島素抵抗的證據。
在肥胖受試者中,門靜脈的平均血漿胰島素濃度是周邊動脈的兩倍多(分別為 34.4 ± 21 和 15.2 ± 8 μU/ml;P = 0.0004)。門靜脈的血漿IL-6濃度比周邊動脈高約50%(表1)。門靜脈的血漿瘦素濃度比周邊動脈低約20%。相比之下,門靜脈的血漿TNF-α、MCP-1、抵抗素和總脂聯素濃度與周邊動脈的濃度無顯著差異。在10名肥胖受試者的子集中評估了門靜脈和橈動脈中以HMW形式存在的脂聯素的百分比;門靜脈(33.1 ± 11%)和動脈(28.6 ± 17%)之間的HMW脂聯素百分比沒有顯著差異。門靜脈IL-6濃度與動脈CRP濃度直接相關(圖1)。
討論
由於獲取門靜脈血液的困難,人類受試者中內臟脂肪釋放的脂肪因子在胰島素抵抗和全身炎症的發病機制中的重要性尚未得到仔細研究。門靜脈負責排空內臟脂肪,是肝臟血液供應的主要來源,約佔肝臟總血流量的80%(15)。在本研究中,我們在患有大量腹內脂肪的重度肥胖受試者進行開放式胃旁路手術期間,從門靜脈和橈動脈獲取了血樣。肥胖受試者的門靜脈血漿IL-6濃度遠高於周邊動脈血,表明內臟脂肪是肥胖人群中IL-6產生的重要來源。這些數據與先前體外研究結果一致,即大網膜比皮下脂肪組織的IL-6分泌更多(16,17)。此外,肥胖受試者的門靜脈IL-6濃度與動脈CRP濃度直接相關。這些結果首次提供了內臟脂肪質量與全身炎症之間潛在機制聯繫的證據。
IL-6直接分泌到門靜脈中會帶來重要的代謝後果,因為IL-6刺激肝臟急性期反應物的產生(18),損害胰島素介導的糖原合成(19),並刺激肝葡萄糖新生(20)。血清IL-6濃度增加也與2型糖尿病和心血管疾病風險增加有關(21-23)。在我們的肥胖受試者中,門靜脈IL-6濃度與全身CRP濃度直接相關,表明IL-6運送到肝臟有助於調節CRP的產生。這一觀察為內臟脂肪與全身胰島素抵抗之間的關係提供了一種潛在機制,並支持內臟脂肪參與調節肝臟急性期反應物的產生,這些反應物激活炎症途徑的可能性(24)。這些發現與從LIKK小鼠獲得的數據一致,顯示肝臟中核因子κB和炎症的局部增加可導致骨骼肌的周邊胰島素抵抗(25)。此外,我們之前發現,在體內,皮下腹部脂肪有大量IL-6的凈釋放,並且在肥胖者中此釋放更多(26)。因此,在基礎條件下,肥胖人士的內臟和皮下脂肪庫都會產生大量循環中的IL-6。
我們受試者的門靜脈血漿瘦素濃度低於周邊動脈血。門靜脈瘦素濃度較低與從隔離脂肪組織獲得的體外數據一致,顯示大網膜比皮下脂肪的ob基因表達和瘦素分泌較低(27,28)。
與IL-6和瘦素不同,其他潛在的炎症性脂肪因子,如TNF-α和抵抗素,其血漿濃度在門靜脈和周邊動脈中相似。這些結果並不令人意外,基於對這些脂肪因子的產生和分解的了解。雖然TNF-α在內臟脂肪中的產生是上調的(29),但這種細胞因子很可能在局部起作用,而不是主要釋放到血液中。事實上,我們之前發現,在體內,皮下腹部脂肪並未有TNF-α的凈釋放(26)。在老鼠中,抵抗素專門由脂肪細胞產生(30),但在人類中,抵抗素的表達在單核細胞和巨噬細胞中更為顯著(31)。脂聯素主要以低分子量六聚體和更大的多聚體高分子量(HMW)複合體形式在血漿中循環(32)。HMW蛋白而不是LMW形式,通過增加肝臟胰島素敏感性和降低肝葡萄糖產生,在肝臟具有直接的有益代謝作用(33-34)。此外,使用重組脂聯素治療已顯示能減少非酒精性脂肪性肝炎小鼠模型中的肝腫大、脂肪變性、肝炎和血清轉氨酶濃度(35)。因此,內臟脂肪對HMW脂聯素的優先產生可能對肝臟具有直接的有益代謝作用,僅通過測量總脂聯素濃度可能無法識別這一點。然而,我們發現門靜脈和全身總脂聯素、HMW或LMW脂聯素濃度之間沒有差異。這些發現表明內臟脂肪不是脂聯素產生的主要部位。
肥胖受試者的空腹血漿胰島素濃度在門靜脈中比周邊動脈血高出兩倍以上,與之前的報告數據一致(36)。這一觀察強調了胰腺和內臟脂肪作為調節肝臟葡萄糖和脂質代謝的內分泌器官的重要戰略解剖位置。這兩種組織均由門靜脈循環排空,這提供了一個高效的系統,可以將蛋白質激素(例如胰島素)和炎症性細胞因子(例如IL-6)直接輸送到肝臟,在那裡它們可以調節內源性葡萄糖產生和急性期炎症反應物的產生。
我們的研究具有幾個重要的限制。首先,它是在受試者的絕收態條件下進行的,這可能無法反映餐後條件下脂肪組織脂肪因子分泌到全身或門靜脈循環的情況。其次,血樣是在手術期間獲得的,這可能影響了血漿脂肪因子濃度。全身麻醉可以減少門靜脈血流(37),這可能增加門靜脈脂肪因子濃度。然而,血流的減少應該同時影響內臟脂肪分泌的所有脂肪因子,但我們發現門靜脈IL-6濃度較高,而瘦素濃度則低於橈動脈血。第三,我們的研究對象僅限於極度肥胖的受試者(BMI >40 kg/m²),他們擁有大量的腹內脂肪。因此,這些結果可能不一定適用於BMI值較低且腹內脂肪較少的肥胖人群。然而,我們並未發現BMI或腰圍與門靜脈或動脈脂肪因子濃度之間有顯著的相關性。第四,我們觀察到的門靜脈IL-6與全身CRP濃度之間的關聯並未證明因果關係。需要進行更多複雜的研究,包括門靜脈輸注IL-6抑制劑和重組門靜脈IL-6,才能證明門靜脈IL-6是CRP產生的主要調節因子。
目前研究的結果支持內臟脂肪是參與肥胖與全身炎症之間複雜相互關係的重要內分泌器官的觀點。我們的發現表明,來自內臟脂肪的IL-6分泌增加進入門靜脈循環,參與了與腹型肥胖相關的全身代謝異常的發病機制。
圖1. 極度肥胖受試者中門靜脈IL-6與全身CRP濃度之間的關係。數據進行了對數轉換。 表1 肥胖受試者中橈動脈與門靜脈血漿脂肪因子濃度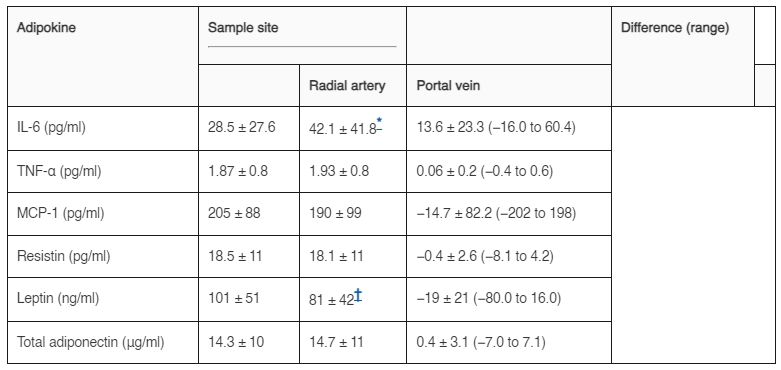
參考文獻
1.Montague CT, O’Rahilly S: The perils of portliness: causes and consequences of visceral adiposity. Diabetes 49: 883–888,2000
2.Despres JP, Moorjani S, Lupien PJ, Tremblay A, Nadeau A, Bouchard C: Regional distribution of body fat, plasma lipoproteins, and cardiovascular disease. Arteriosclerosis 10: 497–511,1990
3.Pouliot MC, Despres JP, Lemieux S, Moorjani S, Bouchard C, Tremblay A, Nadeau A, Lupien PJ: Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol 73: 460–468,1994
4.Zhu S, Wang Z, Heshka S, Heo M, Faith MS, Heymsfield SB: Waist circumference and obesity-associated risk factors among whites in the third National Health and Nutrition Examination Survey: clinical action thresholds. Am J Clin Nutr 76: 743–749,2002
5.Grundy SM, Brewer HB Jr, Cleeman JI, Smith SC Jr, Lenfant C, National Heart, Lung, and Blood Institute, American Heart Association: Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 109: 433–438,2004
6.Boden G: Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes 46: 3–10,1997
7.Nielsen S, Guo ZK, Johnson CM, Hensrud DD, Jensen MD: Splanchnic lipolysis in human obesity. J Clin Invest 113: 1582–1588,2004
8.Klein S: The case of visceral fat: argument for the defense. J Clin Invest 113: 1530–1532,2004
9.Lafontan M: Fat cells: afferent and efferent messages define new approaches to treat obesity. Annu Rev Pharmacol Toxicol 45: 119–146,2004
10.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H: Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112: 1821–1830,2003
11.Park KG, Park KS, Kim MJ, Kim HS, Suh YS, Ahn JD, Park KK, Chang YC, Lee IK: Relationship between serum adiponectin and leptin concentrations and body fat distribution. Diabetes Res Clin Pract 63: 135–142,2004
12.Trujillo ME, Scherer PE: Adiponectin: journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. J Intern Med 257: 167–175,2005
13.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419,1985
14.Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, Engel J, Brownlee M, Scherer PE: Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin: implications fpr metabolic regulation and bioactivity. J Biol Chem 278: 9073–9085,2003
15.Schenk WG Jr, Mcdonald JC, Mcdonald K, Drapanas T: Direct measurement of hepatic blood flow in surgical patients: with related observations on hepatic flow dynamics in experimental animals. Ann Surg 156: 463–471,1962
16.Fried SK, Bunkin DA, Greenberg AS: Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab 83: 847–850,1998
17.Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW: Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology 145: 2273–2282,2004
18.Heinrich PC, Castell JV, Andus T: Interleukin-6 and the acute phase response. Biochem J 265: 621–636,1990
19.Senn JJ, Klover PJ, Nowak IA, Mooney RA: Interleukin-6 induces cellular insulin resistance in hepatocytes. Diabetes 51: 3391–3399,2002
20.Tsigos C, Papanicolaou DA, Kyrou I, Defensor R, Mitsiadis CS, Chrousos GP: Dose dependent effects of recombinant human interleukin-6 on glucose regulation. J Clin Endocrinol Metab 82: 4167–4170,1997
21.Pickup JC, Mattock MB, Chusney GD, Burt D: NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia 40: 1286–1292,1997
22.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM: C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 286: 327–334,2001
23.Plutzky J: Inflammatory pathways in atherosclerosis and acute coronary syndromes. Am J Cardiol 88: 10K–15K,2001
24.Bisoendial RJ, Kastelein JJ, Levels JH, Zwaginga JJ, van den Bogaard B, Reitsma PH, Meijers JC, Hartman D, Levi M, Stroes ES: Activation of inflammation and coagulation after infusion of C-reactive protein in humans. Circ Res 96: 714–716,2005
25.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE: Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med 11: 183–190,2005
26.Mohamed-Ali V, Goodrick SJ, Rawesh A, Katz DR, Miles JM, Yudkin JS, Klein S, Coppack SW: Subcutaneous adipose tissue releases interleukin-6 but not tumor necrosis factor-alpha in vivo. J Clin Endocrinol Metab 82: 4196–4200,1997
27.Ramis JM, Bibiloni B, Moreiro J, Garcia-Sanz JM, Salinas R, Proenza AM, Llado I: Tissue leptin and plasma insulin are associated with lipoprotein lipase activity in severely obese patients. J Nutr Biochem 16: 279–285,2005
28.Van Harmelen V, Reynisdottir S, Eriksson P, Thorne A, Hoffstedt J, Lonnqvist F, Arner P: Leptin secretion from subcutaneous and visceral adipose tissue in women. Diabetes 47: 913–917,1998
29.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM: Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest 95: 2409–2415,1995
30.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA: The hormone resistin links obesity to diabetes. Nature 409: 307–312,2001
31.Patel L, Buckels AC, Kinghorn IJ, Murdock PR, Holbrook JD, Plumpton C, Macphee CH, Smith SA: Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochem Biophys Res Commun 300: 472–476,2003
32.Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, Engel J, Brownlee M, Scherer PE: Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem 279: 12152–12162,2004
33.Cote M, Mauriege P, Bergeron J, Almeras N, Tremblay A, Lemieux I, Despres JP: Adiponectinemia in visceral obesity: impact on glucose tolerance and plasma lipoprotein and lipid levels in men. J Clin Endocrinol Metab 90: 1434–1439,2005
34.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE: The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med 7: 947–953,2001
35.Xu A, Wang Y, Keshaw H, Xu LY, Lam KSL, Cooper GJS: The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver disease in mice. J Clin Invest 112: 91–100,2003
36.Horwitz DL, Starr JI, Mako ME, Blackard WG, Rubenstein AH: Proinsulin, insulin, and C-peptide concentrations in human portal and peripheral blood. J Clin Invest 55: 1278–1283,1975
37.Gelman S: General anesthesia and hepatic circulation. Can J Physiol Pharmacol 65: 1762–1779,1987



 English
English Bahasa Melayu
Bahasa Melayu Bahasa Indonesia
Bahasa Indonesia Tiếng Việt
Tiếng Việt ไทย
ไทย