研究顯示,腦利尿肽(BNP)在心腎相互作用中發揮著關鍵角色,尤其是對抗心力衰竭和慢性腎病。BNP能夠抑制腎素-血管緊張素-醛固酮系統(RAAS),對抗心臟和腎臟疾病的進展。腎髓質的研究表明,腎臟尖端的提取物可能誘導心肌細胞表達和分泌BNP,為心腎疾病的藥物治療提供了新的思路。
本翻譯僅作學術交流用,無商業意圖,請勿轉載,如有疑議問請來信
BNP作為心腎聯繫中的主要參與者
BNP as a Major Player in the Heart-Kidney Connection
by Ryuji Okamoto 1,*,Yusuf Ali 1,Ryotaro Hashizume 2,Noboru Suzuki 3 andMasaaki Ito 1
1Department of Cardiology and Nephrology, Mie University Graduate School of Medicine, 2-174 Edobashi, Tsu, Mie 514-8507, Japan
2Department of Pathology and Matrix Biology, Mie University Graduate School of Medicine, 2-174 Edobashi, Tsu, Mie 514-8507, Japan
3Department of Animal Genomics, Functional Genomics Institute, Mie University Life Science Research Center, 2-174 Edobashi, Tsu, Mie 514-8507, Japan
*
Author to whom correspondence should be addressed.
Int. J. Mol. Sci. 2019, 20(14), 3581; https://doi.org/10.3390/ijms20143581
Submission received: 22 June 2019 / Revised: 15 July 2019 / Accepted: 17 July 2019 / Published: 22 July 2019
(This article belongs to the Special Issue Molecular Basis of Cardiovascular Diseases: Implications of Natriuretic Peptides)
https://www.mdpi.com/1422-0067/20/14/3581
Abstract
Brain natriuretic peptide (BNP) is an important biomarker for patients with heart failure, hypertension and cardiac hypertrophy. Although it is known that BNP levels are relatively higher in patients with chronic kidney disease and no heart disease, the mechanism remains unknown. Here, we review the functions and the roles of BNP in the heart-kidney interaction. In addition, we discuss the relevant molecular mechanisms that suggest BNP is protective against chronic kidney diseases and heart failure, especially in terms of the counterparts of the renin-angiotensin-aldosterone system (RAAS). The renal medulla has been reported to express depressor substances. The extract of the papillary tips from kidneys may induce the expression and secretion of BNP from cardiomyocytes. A better understanding of these processes will help accelerate pharmacological treatments for heart-kidney disease.
Keywords: natriuretic peptide; cardiorenal syndrome; vasopressor; vasodilator; kidney; medulla; renin-angiotensin-aldosterone system
摘要
腦利尿肽(BNP)是心力衰竭、高血壓和心臟肥大患者的重要生物標記。雖然已知慢性腎臟疾病患者且無心臟疾病的BNP水平相對較高,但其機制尚不明確。在這裡,我們回顧了BNP在心腎相互作用中的功能和作用。此外,我們討論了相關的分子機制,表明BNP對慢性腎臟疾病和心力衰竭具有保護作用,特別是在腎素-血管緊張素-醛固酮系統(RAAS)的對應物方面。已有報告指出,腎髓質表達了降壓物質。腎尖提取物可能誘導心肌細胞表達和分泌BNP。對這些過程的更好理解將有助於加速心腎疾病的藥物治療。
引言
腦利尿肽(BNP)是利尿肽(NP)系統的重要組成部分,也被稱為B型NP,主要由心肌細胞在心臟擴張和缺血的反應中分泌,對心腎保護起著重要作用。BNP的腎保護作用包括抑制近曲小管和遠曲小管中的鈉重吸收,通過抑制多種血漿血管收縮劑,改善腎小球濾過率(GFR)和腎血漿流量(RPF)而產生血管擴張作用。此外,BNP輸注還可以抑制心臟和腎臟交感神經張力以及RAAS,並減少內皮素釋放。利尿肽通過與靶細胞表面的特定受體相互作用來介導其功能。目前,已報告了三種不同的利尿肽受體(NPR),包括NPR-A、NPR-B和NPR-C。NPR-A和NPR-B通過激活第二信使循環鸟苷酸單磷酸(cGMP)來刺激鸟苷酸环化酶,介導其效應。在腎臟中,利尿肽引起系統細胞的舒張,增加GFR並減少腎小管中的鈉重吸收。
充血性心力衰竭(CHF)是一種複雜的綜合征,其特點是通過激活不同的神經激素系統,如RAAS和交感神經系統(SNS),以及重要的NP系統來保留鈉和水。幾項實驗和臨床研究已將BNP與CHF中失衡的心腎軸的病理生理學相關聯。在CHF患者中,BNP血漿水平超過100 pg/mL。由於BNP的半衰期較長,已顯示其具有更好的穩定性,並且在診斷上相對於心房NP(ANP)有更好的理解疾病進展的能力,從而改善心血管功能。慢性腎臟疾病(CKD)患者的BNP血漿水平也升高。潛在原因包括腎功能障礙、腎中的降解酶活性降低以及相關的心血管病理生理學。以前的研究報告發現,在腎功能受損的患者中發現了明顯高的血漿BNP水平。
越來越多的證據表明,在慢性心衰、冠狀動脈疾病和/或左心室肥大等方面,與慢性腎病(CKD)患者的關聯性越來越大。最近,心腎綜合症(CRS)這一複雜的病理生理狀況受到了更多關注,它涉及急性心衰(AHF)或慢性心衰(CHF)與腎功能損害之間的聯繫。然而,這種狀況不僅僅是同時存在心臟和腎臟疾病[22]。一項最近的法國前瞻性研究對507名AHF患者進行了研究,結果顯示AHF患者中具有腎功能障礙(CRS患者)的BNP和BNP前激素水平較正常腎功能患者高[23]。
BNP在疾病進展的早期階段作為一種補償性物質,通過誘導利尿和減少RAAS和SNS的作用。與HF或CRS等嚴重疾病狀態類似,盡管BNP水平高,但內源性BNP會變得抗藥性,在這些情況下不再能夠補償容量超載。因此,BNP在對抗這些疾病中激活的RAAS和SNS的不良影響方面的支持作用,為將該肽作為潛在治療藥物提供了理論依據[13,24]。由於已有報告顯示腎髓質表達抑制物質,因此可能可以通過從腎臟的乳突尖端提取物誘導心肌細胞中內源性BNP的表達和分泌[25]。對這些過程的更好理解可能有助於加速CRS的藥理治療。在這裡,我們回顧了BNP在心腎相互作用中的功能和作用。此外,我們還討論了BNP對抗CKD和HF的保護作用的相關分子機制,特別是RAAS系統的相應對應物。
BNP的生化特性
人類BNP mRNA被翻譯成134個氨基酸的前BNP,從中經由丝氨酸蛋白酶corin和/或furin的處理和切割,產生一個生物上不活性的76個氨基酸前BNP(NT-proBNP)和一個活性的32個氨基酸BNP [6,26]。一直以來,人們認為在成熟後,NT-proBNP和BNP以1:1的摩爾比從心臟分泌。然而,最近的研究顯示,在沒有其切割的情況下,也會有一定量的前BNP從心臟分泌出來[27]。因此,當我們看到NT-proBNP升高的患者時,我們應該考慮到部分NT-proBNP升高是由於前BNP的升高造成的。相反,在接受血管緊張素受體/神經激肽酶抑製劑(ARNi)治療的患者中,由於其對BNP降解的抑制作用,BNP會在沒有額外心臟壓力的情況下增加。在本文中,我們將不會在這裡詳細討論它們。NT-proBNP和BNP在心力衰竭的鑑別診斷中同樣有用[28,29],儘管在ARNi治療期間,NT-proBNP和BNP的測定可以提供互補信息[30]。已經詳細審查了BNP的基因結構和後轉錄加工以及循環BNP相關肽的多樣性[2,6,31]。
BNP在腎臟中的功能
BNP在腎臟中發揮重要作用,對腎功能提供多重有益影響[3]。該系統的異常或改變可能促成腎損傷,包括作為其他心血管疾病後果的導致的腎小管損傷[32]。另一方面,BNP血漿水平受腎功能影響,儘管其機制尚不清楚。因此,在腎功能衰竭期間,它們不被認為是理想的血流動力學生物標記[33,34]。在CKD患者中,BNP水平升高可能是心臟釋放增加的結果。在CKD患者中,循環血容量增加,體積超載和動脈僵硬引起的血壓升高,以及心臟肥大和HF等因素都可能導致BNP升高。CKD患者中BNP的升高部分是由於腎臟中BNP的清除受到損害。
NPs的功能是通過它們與靶細胞上的特定表面受體的相互作用而介導的。在腎臟中,BNP通過放鬆系統血管內皮細胞和抑制鈉的管狀部分重吸收來增加GFR [7,35](圖1)。BNP還通過放鬆血管平滑肌細胞減少血管阻力,而與ANP不同,它對血管通透性沒有影響。
圖1. BNP在腎小管中的作用位置。BNP:腦利尿肽。根據Table 1所列出的一系列先驅性研究,探討了BNP在人體內的注射。其中,Jensen等人在1998年對健康男性進行了一項組織有序的BNP輸注研究。在60分鐘的4 pmol/kg/min的劑量後,達到的血漿最大濃度為199 pmol/L(688.6 pg/mL)。作者觀察到尿液流速和腎小球過濾率(分別約增加了60%和5%)增加,並且發現腎素分泌受到抑制(-24%)。此外,鈉的分數排泄增加(+140%)。在輸注後(輸注開始後30至60分鐘),鈉的分數重吸收減少。使用鋰進行清除率測量,鋰只被假設在近曲小管中重吸收,並且與鈉和水具有相同的程度,顯示作用的腎管部位同時發生在近曲小管(-7%)和遠曲腎小管(-5%)。血壓、心率和醛固酮濃度沒有變化。ANF濃度下降(-16%)。這些結果表明,在生理範圍內進行BNP輸注,可以觀察到在健康受試者中增加GFR和抑制鈉排泄,從而增加尿量和鈉排泄,而不影響血壓和心率。這些結果與其他研究在排尿和鈉排泄方面相似,儘管在協議和結果方面存在輕微差異。鈉的重吸收似乎在遠曲腎小管中早於近曲小管減少。有趣的是,在心力衰竭和收縮功能降低(HFrEF)患者中進行BNP輸注試驗(2 pmol/kg/min,60分鐘),由於遠曲腎小管對BNP的反應降低,導致的鈉排泄反應受損,與健康對照組中觀察到的情況相似。此發現可能引人注目,因為這與另一項早期研究的結果一致,該研究發現輕度CKD患者中遠曲腎小管中的鈉重吸收減少早於近曲小管。在遠曲腎小管中,內髓集尿管是ANP的主要靶點。據報導,BNP與ANP共在遠曲小管中局部化,而CNP則觀察到在人體腎臟的近曲小管中。因此,遠曲腎小管中鈉重吸收受損的適應性恢復和降低血壓的可行性表明,如果我們能夠確定CHF或/和CKD患者中BNP的範圍,則這些可能是治療靶點。此外,由於NPR主要表達在腎小管的遠曲部位,這可能是他們在進展期HF中鈉利尿效果受損的另一個可能原因,其中近曲小管的鈉重吸收大大增加。在實驗性CHF模型中,BNP增強了環狀利尿劑的腎利尿和鈉利尿作用,同時還減少了利尿劑誘導的醛固酮產生。
表1. 腦型利尿肽(BNP)輸注對正常受試者和心臟衰竭和高血壓患者的腎功能、腎素-血管緊張素II-醛固酮系統和血流動力學的影響。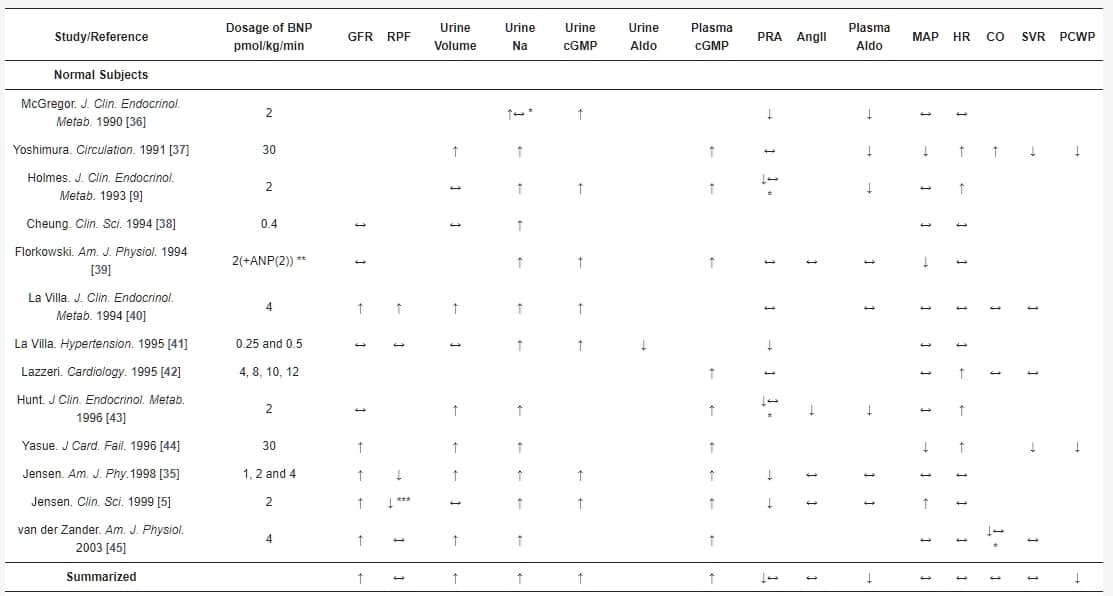
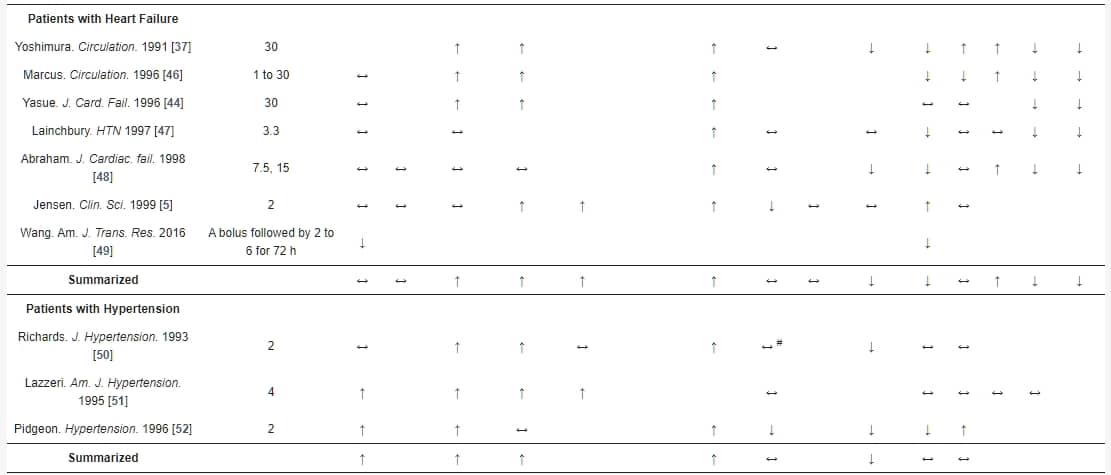
BNP輸注可能在預防CKD的發展中發揮重要作用。 McKie等人最近研究了BNP在心腎功能障礙病理生理學中的作用,並發現在無症狀收縮期HF患者中,持續皮下BNP治療12週可改善腎功能,並對體積擴張產生有利的血流動力學效應[60]。最近的一項研究還調查了早期BNP給藥對CKD患者進行選擇性結紮介入治療或冠狀動脈造影時對造影劑誘導的腎病(CIN)的預防效果[61]。BNP有效地降低了CKD患者CIN的發生率,這表現在改善的估計GFR、胱抑素C和血清肌酸酐與對照組相比。此外,BNP組顯示出更快的恢復。因此,外源性BNP作為CKD患者減輕CIN發生率的預防性藥物[61]。一項回顧性研究分析了尼西酮對328例心力衰竭伴隨保留收縮功能(dHFpEF)患者的腎功能及其臨床安全性的影響,結果得出尼西酮可安全給予,不會對這些患者的長期腎功能產生負面影響[56]。在尼西酮輸注後的1個月,GFR和肌酸酐保持穩定,而在對照組中觀察到明顯的腎功能惡化(GFR和肌酸酐)。此外,他們的多變量分析表明,在1個月時尼西酮是腎功能的重要預測因子[56]。
NPs的血管效應是特定部位的[62]。在腎臟血管中,雖然NPs作為血管擴張劑使輸入動脈擴張,但它們在輸出動脈上則作為血管收縮劑,從而增加GFR[63]。因此,由於假定腎血流可能因外源性NPs而增加、減少或甚至保持不變,這可能會導致相應的關於腎血漿流的不一致結果[13]。
在CKD患者中,受損的腎功能限制了NPs的使用,因為CKD患者無HF的血漿BNP水平升高至約200 pg/mL。這些升高的BNP水平在CKD中是否促進了NP系統的激活並對目標器官產生影響仍然不清楚。腎髓下降調節NPR-A和腎皮質上調節NPR-C的表達,可能是NPs包括BNP在CKD中的抗性的原因,從而導致其在治療HF的CKD患者中的使用受限[64,65]。
BNP在腎臟中的清除
BNP的清除與兩個主要途徑相關。第一個涉及與NPR-C受體的結合,而第二個則涉及由一種稱為神經氨基朊肽酶(NEP)的鋅金屬蛋白酶降解。NPR-C缺乏任何鸞苷酸環化酶活性,並與腺苷酸環化酶抑制相耦合[66],除了ANP和CNP外,在清除BNP方面發揮著重要作用。在人類中,已經證明NPR-C mRNA在多種組織中表達,如心房、腎臟、肺、腸系膜、胎盤、腎上腺、心臟、大腦皮層、小腦和主動脈平滑肌和內皮細胞[67,68]。已經顯示ANP主要在肺、肝和腎臟中降解。BNP與這些器官中的NPR-C的結合能力遠遜於ANP和CNP[69],這表明由於降解量較少,通過NPR-C介導的內化對BNP的清除能力減弱,半衰期較長。BNP還被一種稱為NEP的中性內肽酶降解,NEP主要在腎臟近曲小管的腔內面高水平表達[70],此外還在其他組織如心臟、肺、肝臟和血管平滑肌和內皮細胞中表達。NEP在大鼠腎臟膜中與一種腎臟蛋白酶協作,對BNP進行廣泛降解。然而,在人類腎臟膜中,並非所有的BNP都被NEP降解[71]。此外,NEP介導的BNP降解速度較慢[72]。這些結果表明,人體難以清除腎臟中的BNP。儘管BNP的降解是通過NPR-C和NEP途徑進行的,但在BNP濃度方面,每個過程的確切作用尚未確定[72]。除了NEP外,已報告BNP還被二肽基肽酶-4和胰島素降解酶降解[73,74]。在CHF患者中,觀察到尿中NPs的大幅減少,同時血漿水平升高[75]。然而,另一份報告顯示HF患者新鮮尿液中NT-proBNP增加[76]。
BNP系統與RAA系統
交感神經刺激會激活RAAS,包括增加血管緊張素II(Ang II)和醛固酮的產生,這是由於血管加壓素和去甲腎上腺素血漿水平升高,以及腎素的分泌。SNS和RAAS通過增加心率、收縮能力、前負荷和後負荷來增加心輸出,而這會增加氧耗[77]。通過β-阻滯劑抑制SNS,並通過血管轉化酶(ACE)抑製劑或血管緊張素受體拮抗劑(ARB)以及礦質皮質激素受體拮抗劑對RAAS進行對抗,可能對某些神經荷爾蒙異常不足[78]。作為對容量過載或心臟伸展的反應,來自心臟房室的BNP分泌顯示出心臟作為一種內分泌器官,通過與RAAS、SNS和腎臟互動來維持鹽平衡(圖2)。持續或過度激活的RAAS和SNS導致CHF的發展。此外,BNP也被激活以對抗BNP的利尿、排鈉和血管舒張作用。因此,這抵消了CHF或高血壓中的神經荷爾蒙激活,從而產生有益效果,如利尿作用、血管擴張和抗心臟重塑(圖2)[79,80,81]。
圖2. BNP作為腎素-血管緊張素II-醛固酮系統(RAAS)的對抗調節系統。Aldo,醛固酮;Ang II,血管緊張素II;BP,血壓;BNP,腦型利尿肽;CYP11B2,細胞色素P450家族11亞家族B成員2(醛固酮合成酶);cGMP,環磷酸鸟苷單磷酸;GFR,腎小球過濾率;NPR-A,利尿肽受體A。研究顯示,在影響腎血流或腎小球過濾率之前,BNP已直接抑制了來自腎臟的腎素的產生[82],類似於ANP [83]。這一結果表明,BNP直接抑制了由鹽過度攝入激活的腎小球管腔反饋反應。
使用大鼠心肌細胞的實驗性研究報告指出,無論是內源性還是外源性的BNP都會降低醛固酮合成酶(CYP11B2)mRNA的表達,這可能導致RAAS的抑制以及心肌肥大和纖維化的減弱[84]。此外,在培養的原代人腎上腺皮質細胞中,報告稱BNP對抗Ang II刺激的醛固酮生物合成,這是由於CYP11B2和CYP11B1的表達減少,這是醛固酮的最重要的合成酶[85]。相反,BNP未能直接抑制腎上腺髓質細胞中的優勢胺類的產生和酪氨酸羥化酶的合成,後者是多巴胺的合成酶 [86]。BNP輸注在正常受試者中誘導了交感神經抑制作用,並抑制了CHF患者的腎交感神經活動,但在健康受試者中沒有 [8]。然而,另一項研究發現,在對HF患者進行BNP輸注後,系統和右側心壓降低,而腎素、醛固酮和去甲腎上腺素血漿水平沒有變化,這表明BNP存在RAAS或SNS獨立的直接血管舒張作用 [47]。這兩個系統彼此影響,BNP通過cGMP抑制腎素分泌和CYP11B2表達,從而對抗RAAS,而RAAS阻塞則反過來激活BNP。這表明這些在HF中可能產生協同作用 [87]。因此,利尿肽明顯與RAAS相互作用,在某些生理條件下與血漿Ang II水平呈反比 [88]。此外,由於HF中兩個激素系統的血漿水平增加,這表明它們相互平衡 [89]。
利尿肽增強聯合RAAS阻斷:雙作用血管緊張素受體/神經胜肽酶抑製劑(ARNi)
盡管在HF中BNP逐漸被激活,但其反應往往不足以抵消RAAS和SNS的激活所導致的鈉滯留和血管收縮。因此,在試圖最小化這些神經激素系統的失調方面,進行了更深入的實驗和臨床方法。目前促進NP的策略包括合成或使用激動劑增加其生物活性,以及抑製NEP以減少其降解 [90,91]。Nesiritide是一種重組BNP,於2001年獲得美國食品和藥物管理局(FDA)批准,已被證明在HF管理中促進臨床改善 [92]。然而,據一項荟萃分析報告,它被報導會惡化腎功能並增加死亡率 [93]。此外,嚴重的低血壓和短半衰期使這些制劑,包括尼西酮、卡匹利酮和烏拉利酮,在臨床上不完美。僅使用NEP抑製劑(NEPi)會導致RAAS的激活並減弱Ang II的降解。再次,NEPi和ACE抑製劑的組合易使患者發生血管水腫的高風險 [94]。因此,使用ARB是與NEPi一起使用RAAS抑制的最佳方法。第一個血管緊張素受體-神經胜肽酶抑制劑(ARNI,LCZ696),通過將ARB(缬沙坦)與NEPi(沙库比替林)結合而開發,是HF治療中的一個重大進步 [95,96]。沙库比替林和缬沙坦的組合增強了NP的有益效果,抑制了Ang II的有害效果。它保留了ACE機制對激肽的降解並保護免受血管水腫的形成 [97]。動物研究表明,NEPi和RAAS抑制劑的組合減少了蛋白尿並預防了腎損傷 [98],同時改善了心臟重塑、纖維化和肥大 [99]。最近的英國HARP-III(英國心臟和腎保護-III)研究對414名eGFR為20至60 mL/min/1.73 m2的CKD患者進行了研究,結果顯示在12個月內,沙库比替林和缬沙坦的組合耐受良好,對腎功能和尿白蛋白尿的影響與ARB缬沙坦相似,具有降壓和心臟生物標記物降低效果,表明該組合可能有潛力降低CKD的心血管風險 [100]。
LCZ696在高血壓患者中降低了血壓的效果比缬沙坦更有效 [101]。PARAMOUNT(心臟收縮功能保留型HF的前瞻性ARNI與ARB比較試驗)試驗表明,與缬沙坦相比,LCZ696改善了整體臨床狀態,降低了心房壓力,並提高了GFR [102]。在收縮功能降低的HF患者中,LCZ696據報比依那普利更有效地減少住院率、心血管和猝死,防止HF進展,改善腎功能,以及生活質量,在前瞻性ARNI與ACE抑製劑比較研究中 [103,104,105,106]。基於這項試驗的眾多有利結果,LCZ696已於2015年獲得FDA批准用於治療HFrEF。ARNI在幾項研究中,包括PARADIGM-HF試驗,與HF中RAAS抑制劑相比,改善了腎功能 [102,106,107]。最近,一項使用小鼠心肌梗死(MI)模型和體外小鼠腹腔巨噬細胞的實驗研究表明,LCZ696與RAA和NP系統之間的平衡更好,並在MI後減輕了心臟破裂,表明LCZ696通過其雙重調節機制抑制了巨噬細胞的炎症和降解反應,早期治療LCZ696可能具有心臟保護作用 [108]。
盡管迄今為止ARNi具有眾多好處,但仍存在一些限制其臨床使用的問題,例如ARB導致激肽素水平升高,引起血管水腫 [109],以及通過阻斷β-淀粉样蛋白的分解而誘發阿爾茨海默病的可能性 [110]。值得注意的是,由於沙库比替林在患有腎功能障礙的患者中主要是通過腎排泄,因此它可能會在輕度低血壓患者中積累並誘發低血壓的發生 [74]。
腎臟衍生的血管收縮素和血管舒張素
腎臟在調節血壓方面發揮著重要作用。除了通過平衡水分和電解質排泄進行容積控制外,腎臟還擁有幾種用於控制血壓的物質 [111](見圖3)。最著名的物質來自腎皮質。Tigerstedt和Bergman證明,將來自兔子腎臟皮質的提取物注射到受體兔中導致了血壓升高,從而隨後發現了血管收縮素,一種強有力的血管收縮素 [112]。
圖3. 血管收縮素和血管舒張素的腎臟衍生物。ET-1,內皮素1;PGE2,前列腺素E2。內皮素-1(ET-1)是一種由內皮細胞、腎小管細胞和內髓集合管細胞產生的21個氨基酸的血管收縮素 [113]。在生理狀態下,鹽攝入增加會誘導腎小管產生ET-1,它抑制上皮細胞鈉通道,導致鈉重吸收率降低,從而導致尿鈉排泄增加。然而,在病理狀態下,ET-1被認為是一種血管收縮素,因為它會導致腎小球和間質腎臟疾病以及血管疾病的發展 [114]。
前列腺素(PG)是腎臟中涉及各種生理和病理過程的脂質介質 [115]。PGE2是最豐富的腎臟花生四烯酸代謝物。PGE2由微粒體PGE合成酶在髓細密部、遠曲小管、集尿管和腎髓間質細胞(RMIC)中產生 [116]。儘管仍然存在著矛盾的結論,但通常認為PGE2是一種血管舒張素,因其利尿作用 [117]。
組織激肽原主要在顎下腺、胰臟和腎臟中表達。激肽原主要在遠曲小管 [118,119]、皮質集合小管 [119,120] 中產生,可能還在內髓集合小管中產生 [120]。組織激肽原從低分子和高分子量激肽原中釋放激肽。激肽被激肽酶水解,最著名的激肽酶是血管收縮素轉化酶(ACE)。在人類中,組織激肽原釋放Lys-激肽(激肽)。激肽-激肽系統增加了腎臟血流,從而導致更多的尿液排泄、水分和鈉排泄 [121],與其對遠曲小管的直接作用相互配合 [122]。有報告顯示,尿激肽酶的減少與高血壓的發展有關 [123,124,125]。因此,激肽原被認為是一種血管舒張素。
幾個獨立小組報告稱,腎髓具有一種作為RAAS對應物的降壓物質 [126,127]。這種降壓劑是由RMIC產生的。RMIC含有細胞內分泌顆粒,其大小與血壓和腎血流成正比 [128]。儘管尚未正確識別出這些包涵物,但電子顯微鏡研究顯示其成分包括游離脂肪酸和PG。然而,這種降壓物質似乎不是PG、一氧化氮或血小板活化因子 [126,129]。在單腎單結扎高血壓大鼠模型中,腎尖部RMIC在腎臟結扎時具有豐富的顆粒,並且在解除結扎後已被證明是顆粒脫顆粒 [130]。目前尚不清楚腎尖部是否含有與研究人員一直在試圖鑒定的腎髓中相同的物質,因為大多數研究人員未將腎尖部與整個內髓區分開來。
腎尖部可能對心肌細胞中 BNP 的表達有所貢獻
最近,我們開發了一種BNP報告小鼠,其在1136 bp的小鼠NPPB基因的片段上帶有tdTomato,從−1000到+136,並證明了該啟動子在腎尖部特異性激活,並且未伴隨BNP mRNA的表達 [25]。未找到證據顯示存在BNP異構體或其他核苷酸表達,除了BNP和tdTomato。在用腎尖提取物處理後,新生兒心肌細胞的表達和分泌BNP意外地增加。儘管由於污染而可能產生人工製品,但我們發現腎尖部和腎臟其他部位之間的Ang II、ET-1以及A、B和C型NP的表達沒有變化。儘管其機制尚不清楚,但我們最初對老年雌性小鼠進行了評估,因為老化和女性性別對正常人和心衰患者的BNP表達有所貢獻。但是,我們觀察到較年輕成年人和/或雄性成年小鼠的腎尖部中BNP啟動子的類似活化,儘管這在新生兒小鼠中未被認識到 [25]。
pBNP-tdTomato陽性細胞是間質細胞,並且不具有增殖能力。據報導,腎髓擁有降低血壓的能力,因為其血管擴張活性 [126,127]。為了評估來自腎臟的腎尖部的這種活性,我們將腎尖提取物通過腹腔注射到易患中風的自發性高血壓大鼠(SHR-SPs)中。腹腔注射腎尖提取物將血壓從210 mmHg降至165 mmHg,並且伴隨著SHR-SP大鼠血清BNP和尿cGMP產量的增加。此外,與由於心肌梗死而導致心衰的大鼠相比,用腎尖提取物治療顯著誘導了心肌細胞中的BNP表達 [25]。
結論
BNP 在心腎聯繫中扮演著重要角色,通過其對 RAAS 的抑制作用,特別是在心臟和腎臟中。腎臟除了通過排尿進行體積控制外,還擁有幾種參與調節血壓的物質。此外,腎尖部可能在調節心肌細胞中的 BNP 表達中發揮重要作用。需要進行更多的研究,以確定腎臟降壓系統與 BNP 調節之間的關係,特別是在心血管疾病(如心力衰竭、高血壓和 CKD)方面。
參考文獻
- Sudoh, T.; Kangawa, K.; Minamino, N.; Matsuo, H. A new natriuretic peptide in porcine brain. Nature 1988, 332, 78–81. [Google Scholar] [CrossRef] [PubMed]
- LaPointe, M.C. Molecular regulation of the brain natriuretic peptide gene. Peptides 2005, 26, 944–956. [Google Scholar] [CrossRef] [PubMed]
- Volpe, M. Natriuretic peptides and cardio-renal disease. Int. J. Cardiol. 2014, 176, 630–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabatine, M.S.; Morrow, D.A.; de Lemos, J.A.; Omland, T.; Desai, M.Y.; Tanasijevic, M.; Hall, C.; McCabe, C.H.; Braunwald, E. Acute changes in circulating natriuretic peptide levels in relation to myocardial ischemia. J. Am. Coll. Cardiol. 2004, 44, 1988–1995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, K.T.; Eiskjaer, H.; Carstens, J.; Pedersen, E.B. Renal effects of brain natriuretic peptide in patients with congestive heart failure. Clin. Sci. 1999, 96, 5–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishikimi, T.; Kuwahara, K.; Nakao, K. Current biochemistry, molecular biology, and clinical relevance of natriuretic peptides. J. Cardiol. 2011, 57, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Levin, E.R.; Gardner, D.G.; Samson, W.K. Natriuretic peptides. N. Engl. J. Med. 1998, 339, 321–328. [Google Scholar]
- Brunner-La Rocca, H.P.; Kaye, D.M.; Woods, R.L.; Hastings, J.; Esler, M.D. Effects of intravenous brain natriuretic peptide on regional sympathetic activity in patients with chronic heart failure as compared with healthy control subjects. J. Am. Coll. Cardiol. 2001, 37, 1221–1227. [Google Scholar] [CrossRef] [Green Version]
- Holmes, S.J.; Espiner, E.A.; Richards, A.M.; Yandle, T.G.; Frampton, C. Renal, endocrine, and hemodynamic effects of human brain natriuretic peptide in normal man. J. Clin. Endocrinol. Metab. 1993, 76, 91–96. [Google Scholar]
- Kohno, M.; Yokokawa, K.; Horio, T.; Yasunari, K.; Murakawa, K.; Takeda, T. Atrial and brain natriuretic peptides inhibit the endothelin-1 secretory response to angiotensin II in porcine aorta. Circ. Res. 1992, 70, 241–247. [Google Scholar] [CrossRef]
- De Arriba, G.; Barrio, V.; Olivera, A.; Rodriguez-Puyol, D.; Lopez-Novoa, J.M. Atrial natriuretic peptide inhibits angiotensin II-induced contraction of isolated glomeruli and cultured glomerular mesangial cells of rats: The role of calcium. J. Lab. Clin. Med. 1988, 111, 466–474. [Google Scholar] [PubMed]
- Ballermann, B.J.; Hoover, R.L.; Karnovsky, M.J.; Brenner, B.M. Physiologic regulation of atrial natriuretic peptide receptors in rat renal glomeruli. J. Clin. Investig. 1985, 76, 2049–2056. [Google Scholar] [CrossRef] [PubMed]
- Houben, A.J.; van der Zander, K.; de Leeuw, P.W. Vascular and renal actions of brain natriuretic peptide in man: Physiology and pharmacology. Fundam. Clin. Pharmacol. 2005, 19, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Doust, J.; Lehman, R.; Glasziou, P. The role of BNP testing in heart failure. Am. Fam. Physician 2006, 74, 1893–1898. [Google Scholar] [PubMed]
- Brenner, B.M.; Ballermann, B.J.; Gunning, M.E.; Zeidel, M.L. Diverse biological actions of atrial natriuretic peptide. Physiol. Rev. 1990, 70, 665–699. [Google Scholar] [CrossRef] [PubMed]
- Rademaker, M.T.; Richards, A.M. Cardiac natriuretic peptides for cardiac health. Clin. Sci. 2005, 108, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Semenov, A.G.; Katrukha, A.G. Analytical Issues with Natriuretic Peptides—Has this been Overly Simplified? EJIFCC 2016, 27, 189–207. [Google Scholar]
- Kitakaze, M.; Asakura, M.; Kim, J.; Shintani, Y.; Asanuma, H.; Hamasaki, T.; Seguchi, O.; Myoishi, M.; Minamino, T.; Ohara, T.; et al. Human atrial natriuretic peptide and nicorandil as adjuncts to reperfusion treatment for acute myocardial infarction (J-WIND): Two randomised trials. Lancet 2007, 370, 1483–1493. [Google Scholar] [CrossRef]
- Spanaus, K.S.; Kronenberg, F.; Ritz, E.; Schlapbach, R.; Fliser, D.; Hersberger, M.; Kollerits, B.; Konig, P.; von Eckardstein, A. B-type natriuretic peptide concentrations predict the progression of nondiabetic chronic kidney disease: The Mild-to-Moderate Kidney Disease Study. Clin. Chem. 2007, 53, 1264–1272. [Google Scholar] [CrossRef]
- Van Kimmenade, R.R.; Januzzi, J.L., Jr.; Bakker, J.A.; Houben, A.J.; Rennenberg, R.; Kroon, A.A.; Crijns, H.J.; van Dieijen-Visser, M.P.; de Leeuw, P.W.; Pinto, Y.M. Renal clearance of B-type natriuretic peptide and amino terminal pro-B-type natriuretic peptide a mechanistic study in hypertensive subjects. J. Am. Coll. Cardiol. 2009, 53, 884–890. [Google Scholar] [CrossRef]
- Takase, H.; Dohi, Y. Kidney function crucially affects B-type natriuretic peptide (BNP), N-terminal proBNP and their relationship. Eur. J. Clin. Investig. 2014, 44, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Niizuma, S.; Iwanaga, Y.; Yahata, T.; Miyazaki, S. Renocardiovascular Biomarkers: From the Perspective of Managing Chronic Kidney Disease and Cardiovascular Disease. Front. Cardiovasc. Med. 2017, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis, D.; Fraticelli, L.; Bassand, A.; Manzo-Silberman, S.; Peschanski, N.; Charpentier, S.; Elbaz, M.; Savary, D.; Bonnefoy-Cudraz, E.; Laribi, S.; et al. Impact of renal dysfunction on the management and outcome of acute heart failure: Results from the French prospective, multicentre, DeFSSICA survey. BMJ Open 2019, 9, e022776. [Google Scholar] [CrossRef] [PubMed]
- Volpe, M.; Carnovali, M.; Mastromarino, V. The natriuretic peptides system in the pathophysiology of heart failure: From molecular basis to treatment. Clin. Sci. 2016, 130, 57–77. [Google Scholar] [CrossRef] [PubMed]
- Goto, I.; Okamoto, R.; Hashizume, R.; Suzuki, N.; Ito, R.; Yamanaka, K.; Saito, H.; Kiyonari, H.; Tawara, I.; Kageyama, Y.; et al. Renal papillary tip extract stimulates BNP production and excretion from cardiomyocytes. PLoS ONE 2018, 13, e0197078. [Google Scholar] [CrossRef] [PubMed]
- Clerico, A.; Passino, C.; Franzini, M.; Emdin, M. Cardiac biomarker testing in the clinical laboratory: Where do we stand? General overview of the methodology with special emphasis on natriuretic peptides. Clin. Chim. Acta 2015, 443, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Waldo, S.W.; Beede, J.; Isakson, S.; Villard-Saussine, S.; Fareh, J.; Clopton, P.; Fitzgerald, R.L.; Maisel, A.S. Pro-B-type natriuretic peptide levels in acute decompensated heart failure. J. Am. Coll. Cardiol. 2008, 51, 1874–1882. [Google Scholar] [CrossRef]
- Santaguida, P.L.; Don-Wauchope, A.C.; Oremus, M.; McKelvie, R.; Ali, U.; Hill, S.A.; Balion, C.; Booth, R.A.; Brown, J.A.; Bustamam, A.; et al. BNP and NT-proBNP as prognostic markers in persons with acute decompensated heart failure: A systematic review. Heart Fail. Rev. 2014, 19, 453–470. [Google Scholar] [CrossRef]
- Mueller, T.; Gegenhuber, A.; Poelz, W.; Haltmayer, M. Head-to-head comparison of the diagnostic utility of BNP and NT-proBNP in symptomatic and asymptomatic structural heart disease. Clin. Chim. Acta 2004, 341, 41–48. [Google Scholar] [CrossRef]
- Clerico, A.; Zaninotto, M.; Passino, C.; Plebani, M. New issues on measurement of B-type natriuretic peptides. Clin. Chem. Lab. Med. 2017, 56, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Kuwahara, K.; Nakagawa, Y.; Nishikimi, T. Cutting Edge of Brain Natriuretic Peptide (BNP) Research- The Diversity of BNP Immunoreactivity and Its Clinical Relevance. Circ. J. 2018, 82, 2455–2461. [Google Scholar] [CrossRef] [PubMed]
- Rubattu, S.; Forte, M.; Marchitti, S.; Volpe, M. Molecular Implications of Natriuretic Peptides in the Protection from Hypertension and Target Organ Damage Development. Int. J. Mol. Sci. 2019, 20, 798. [Google Scholar] [CrossRef] [PubMed]
- Santos-Araujo, C.; Leite-Moreira, A.; Pestana, M. Clinical value of natriuretic peptides in chronic kidney disease. Nefrologia 2015, 35, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Vanderheyden, M.; Bartunek, J.; Filippatos, G.; Goethals, M.; Vlem, B.V.; Maisel, A. Cardiovascular disease in patients with chronic renal impairment: Role of natriuretic peptides. Congest. Heart Fail. 2008, 14, 38–42. [Google Scholar] [CrossRef]
- Jensen, K.T.; Carstens, J.; Pedersen, E.B. Effect of BNP on renal hemodynamics, tubular function and vasoactive hormones in humans. Am. J. Physiol. 1998, 274, F63–F72. [Google Scholar] [CrossRef] [PubMed]
- McGregor, A.; Richards, M.; Espiner, E.; Yandle, T.; Ikram, H. Brain natriuretic peptide administered to man: Actions and metabolism. J. Clin. Endocrinol. Metab. 1990, 70, 1103–1107. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, M.; Yasue, H.; Morita, E.; Sakaino, N.; Jougasaki, M.; Kurose, M.; Mukoyama, M.; Saito, Y.; Nakao, K.; Imura, H. Hemodynamic, renal, and hormonal responses to brain natriuretic peptide infusion in patients with congestive heart failure. Circulation 1991, 84, 1581–1588. [Google Scholar] [CrossRef]
- Cheung, B.M.; Dickerson, J.E.; Ashby, M.J.; Brown, M.J.; Brown, J. Effects of physiological increments in human alpha-atrial natriuretic peptide and human brain natriuretic peptide in normal male subjects. Clin. Sci. 1994, 86, 723–730. [Google Scholar] [CrossRef]
- Florkowski, C.M.; Richards, A.M.; Espiner, E.A.; Yandle, T.G.; Frampton, C. Renal, endocrine, and hemodynamic interactions of atrial and brain natriuretic peptides in normal men. Am. J. Physiol. 1994, 266, R1244–R1250. [Google Scholar] [CrossRef]
- La Villa, G.; Fronzaroli, C.; Lazzeri, C.; Porciani, C.; Bandinelli, R.; Vena, S.; Messeri, G.; Franchi, F. Cardiovascular and renal effects of low dose brain natriuretic peptide infusion in man. J. Clin. Endocrinol. Metab. 1994, 78, 1166–1171. [Google Scholar]
- La Villa, G.; Stefani, L.; Lazzeri, C.; Zurli, C.; Guerra, C.T.; Barletta, G.; Bandinelli, R.; Strazzulla, G.; Franchi, F. Acute effects of physiological increments of brain natriuretic peptide in humans. Hypertension 1995, 26, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Lazzeri, C.; La Villa, G.; Bisi, G.; Boddi, V.; Messeri, G.; Strazzulla, G.; Franchi, F. Cardiovascular function during brain natriuretic peptide infusion in man. Cardiology 1995, 86, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Hunt, P.J.; Espiner, E.A.; Nicholls, M.G.; Richards, A.M.; Yandle, T.G. Differing biological effects of equimolar atrial and brain natriuretic peptide infusions in normal man. J. Clin. Endocrinol. Metab. 1996, 81, 3871–3876. [Google Scholar] [PubMed]
- Yasue, H.; Yoshimura, M. Natriuretic peptides in the treatment of heart failure. J. Card. Fail. 1996, 2, S277–S285. [Google Scholar] [CrossRef]
- Van der Zander, K.; Houben, A.J.; Hofstra, L.; Kroon, A.A.; de Leeuw, P.W. Hemodynamic and renal effects of low-dose brain natriuretic peptide infusion in humans: A randomized, placebo-controlled crossover study. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H1206–H1212. [Google Scholar] [CrossRef] [PubMed]
- Marcus, L.S.; Hart, D.; Packer, M.; Yushak, M.; Medina, N.; Danziger, R.S.; Heitjan, D.F.; Katz, S.D. Hemodynamic and renal excretory effects of human brain natriuretic peptide infusion in patients with congestive heart failure. A double-blind, placebo-controlled, randomized crossover trial. Circulation 1996, 94, 3184–3189. [Google Scholar] [CrossRef] [PubMed]
- Lainchbury, J.G.; Richards, A.M.; Nicholls, M.G.; Hunt, P.J.; Ikram, H.; Espiner, E.A.; Yandle, T.G.; Begg, E. The effects of pathophysiological increments in brain natriuretic peptide in left ventricular systolic dysfunction. Hypertension 1997, 30, 398–404. [Google Scholar] [CrossRef]
- Abraham, W.T.; Lowes, B.D.; Ferguson, D.A.; Odom, J.; Kim, J.K.; Robertson, A.D.; Bristow, M.R.; Schrier, R.W. Systemic hemodynamic, neurohormonal, and renal effects of a steady-state infusion of human brain natriuretic peptide in patients with hemodynamically decompensated heart failure. J. Card. Fail. 1998, 4, 37–44. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, X.; Fan, W.; Fan, Y.; Li, W.; Fu, X. Effects of recombinant human brain natriuretic peptide on renal function in patients with acute heart failure following myocardial infarction. Am. J. Transl. Res. 2016, 8, 239–245. [Google Scholar]
- Richards, A.M.; Crozier, I.G.; Holmes, S.J.; Espiner, E.A.; Yandle, T.G.; Frampton, C. Brain natriuretic peptide: Natriuretic and endocrine effects in essential hypertension. J. Hypertens. 1993, 11, 163–170. [Google Scholar] [CrossRef]
- Lazzeri, C.; Franchi, F.; Porciani, C.; Fronzaroli, C.; Casini Raggi, V.; De Feo, M.L.; Mannelli, M.; Cersosimo, R.M.; La Villa, G. Systemic hemodynamics and renal function during brain natriuretic peptide infusion in patients with essential hypertension. Am. J. Hypertens. 1995, 8, 799–807. [Google Scholar] [CrossRef]
- Pidgeon, G.B.; Richards, A.M.; Nicholls, M.G.; Espiner, E.A.; Yandle, T.G.; Frampton, C. Differing metabolism and bioactivity of atrial and brain natriuretic peptides in essential hypertension. Hypertension 1996, 27, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Kamper, A.L.; Holstein-Rathlou, N.H.; Leyssac, P.P.; Strandgaard, S. Lithium clearance in chronic nephropathy. Clin. Sci. 1989, 77, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Totsune, K.; Takahashi, K.; Murakami, O.; Satoh, F.; Sone, M.; Saito, T.; Sasano, H.; Mouri, T.; Abe, K. Natriuretic peptides in the human kidney. Hypertension 1994, 24, 758–762. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, S.S.; Stebbins, A.; Voors, A.A.; Hasselblad, V.; Ezekowitz, J.A.; Califf, R.M.; O’Connor, C.M.; Starling, R.C.; Hernandez, A.F. Effects of nesiritide and predictors of urine output in acute decompensated heart failure: Results from ASCEND-HF (acute study of clinical effectiveness of nesiritide and decompensated heart failure). J. Am. Coll. Cardiol. 2013, 62, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Kelesidis, I.; Mazurek, J.; Khullar, P.; Saeed, W.; Vittorio, T.; Zolty, R. The effect of nesiritide on renal function and other clinical parameters in patients with decompensated heart failure and preserved ejection fraction. Congest. Heart Fail. 2012, 18, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Fontoura, B.M.; Nussenzveig, D.R.; Pelton, K.M.; Maack, T. Atrial natriuretic factor receptors in cultured renomedullary interstitial cells. Am. J. Physiol. 1990, 258, C692–C699. [Google Scholar] [CrossRef] [PubMed]
- Verbrugge, F.H.; Dupont, M.; Steels, P.; Grieten, L.; Swennen, Q.; Tang, W.H.; Mullens, W. The kidney in congestive heart failure: ‘Are natriuresis, sodium, and diuretics really the good, the bad and the ugly?’. Eur. J. Heart Fail. 2014, 16, 133–142. [Google Scholar] [CrossRef]
- Cataliotti, A.; Boerrigter, G.; Costello-Boerrigter, L.C.; Schirger, J.A.; Tsuruda, T.; Heublein, D.M.; Chen, H.H.; Malatino, L.S.; Burnett, J.C., Jr. Brain natriuretic peptide enhances renal actions of furosemide and suppresses furosemide-induced aldosterone activation in experimental heart failure. Circulation 2004, 109, 1680–1685. [Google Scholar] [CrossRef]
- McKie, P.M.; Schirger, J.A.; Benike, S.L.; Harstad, L.K.; Slusser, J.P.; Hodge, D.O.; Redfield, M.M.; Burnett, J.C., Jr.; Chen, H.H. Chronic subcutaneous brain natriuretic peptide therapy in asymptomatic systolic heart failure. Eur. J. Heart Fail. 2016, 18, 433–441. [Google Scholar] [CrossRef]
- Liu, J.; Xie, Y.; He, F.; Gao, Z.; Hao, Y.; Zu, X.; Chang, L.; Li, Y. Recombinant Brain Natriuretic Peptide for the Prevention of Contrast-Induced Nephropathy in Patients with Chronic Kidney Disease Undergoing Nonemergent Percutaneous Coronary Intervention or Coronary Angiography: A Randomized Controlled Trial. Biomed Res. Int. 2016, 2016, 5985327. [Google Scholar] [CrossRef] [PubMed]
- Woods, R.L.; Jones, M.J. Atrial, B-type, and C-type natriuretic peptides cause mesenteric vasoconstriction in conscious dogs. Am. J. Physiol. 1999, 276, R1443–R1452. [Google Scholar] [CrossRef] [PubMed]
- Marin-Grez, M.; Fleming, J.T.; Steinhausen, M. Atrial natriuretic peptide causes pre-glomerular vasodilatation and post-glomerular vasoconstriction in rat kidney. Nature 1986, 324, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Santos-Araujo, C.; Roncon-Albuquerque, R., Jr.; Moreira-Rodrigues, M.; Henriques-Coelho, T.; Quelhas-Santos, J.; Faria, B.; Sampaio-Maia, B.; Leite-Moreira, A.F.; Pestana, M. Local modulation of the natriuretic peptide system in the rat remnant kidney. Nephrol. Dial. Transplant. 2009, 24, 1774–1782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sackner-Bernstein, J.D.; Skopicki, H.A.; Aaronson, K.D. Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure. Circulation 2005, 111, 1487–1491. [Google Scholar] [CrossRef] [PubMed]
- Anand-Srivastava, M.B. Natriuretic peptide receptor-C signaling and regulation. Peptides 2005, 26, 1044–1059. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.G.; Arfsten, A.; Fuller, F.; Miller, J.A.; Gregory, L.C.; Lewicki, J.A. Isolation and functional expression of the human atrial natriuretic peptide clearance receptor cDNA. Biochem. Biophys. Res. Commun. 1990, 171, 796–803. [Google Scholar] [CrossRef]
- Wilcox, J.N.; Augustine, A.; Goeddel, D.V.; Lowe, D.G. Differential regional expression of three natriuretic peptide receptor genes within primate tissues. Mol. Cell. Biol. 1991, 11, 3454–3462. [Google Scholar] [CrossRef]
- He, X.; Chow, D.; Martick, M.M.; Garcia, K.C. Allosteric activation of a spring-loaded natriuretic peptide receptor dimer by hormone. Science 2001, 293, 1657–1662. [Google Scholar] [CrossRef]
- Jalal, F.; Dehbi, M.; Berteloot, A.; Crine, P. Biosynthesis and polarized distribution of neutral endopeptidase in primary cultures of kidney proximal tubule cells. Biochem. J. 1994, 302, 669–674. [Google Scholar] [CrossRef] [Green Version]
- Dickey, D.M.; Potter, L.R. Human B-type natriuretic peptide is not degraded by meprin A. Biochem. Pharmacol. 2010, 80, 1007–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, M.W.; Espiner, E.A.; Yandle, T.G.; Charles, C.J.; Richards, A.M. Delayed metabolism of human brain natriuretic peptide reflects resistance to neutral endopeptidase. J. Endocrinol. 2000, 167, 239–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ralat, L.A.; Guo, Q.; Ren, M.; Funke, T.; Dickey, D.M.; Potter, L.R.; Tang, W.J. Insulin-degrading enzyme modulates the natriuretic peptide-mediated signaling response. J. Biol. Chem. 2011, 286, 4670–4679. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Ping, P.; Wang, F.; Luo, L. Synthesis, secretion, function, metabolism and application of natriuretic peptides in heart failure. J. Biol. Eng. 2018, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- Linssen, G.C.; Damman, K.; Hillege, H.L.; Navis, G.; van Veldhuisen, D.J.; Voors, A.A. Urinary N-terminal prohormone brain natriuretic peptide excretion in patients with chronic heart failure. Circulation 2009, 120, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Jungbauer, C.G.; Buchner, S.; Birner, C.; Resch, M.; Heinicke, N.; Debl, K.; Buesing, M.; Biermeier, D.; Schmitz, G.; Riegger, G.; et al. N-terminal pro-brain natriuretic peptide from fresh urine for the biochemical detection of heart failure and left ventricular dysfunction. Eur. J. Heart Fail. 2010, 12, 331–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, P.C.; Guo, J.; Zhang, A. The renal and cardiovascular effects of natriuretic peptides. Adv. Physiol. Educ. 2017, 41, 179–185. [Google Scholar] [CrossRef] [Green Version]
- Volpe, M.; Battistoni, A.; Mastromarino, V. Natriuretic peptides and volume handling in heart failure: The paradigm of a new treatment. Eur. J. Heart Fail. 2016, 18, 442–444. [Google Scholar] [CrossRef]
- Von Lueder, T.G.; Sangaralingham, S.J.; Wang, B.H.; Kompa, A.R.; Atar, D.; Burnett, J.C., Jr.; Krum, H. Renin-angiotensin blockade combined with natriuretic peptide system augmentation: Novel therapeutic concepts to combat heart failure. Circ. Heart Fail. 2013, 6, 594–605. [Google Scholar] [CrossRef]
- Corti, R.; Burnett, J.C., Jr.; Rouleau, J.L.; Ruschitzka, F.; Luscher, T.F. Vasopeptidase inhibitors: A new therapeutic concept in cardiovascular disease? Circulation 2001, 104, 1856–1862. [Google Scholar] [CrossRef]
- Tokudome, T.; Kishimoto, I.; Horio, T.; Arai, Y.; Schwenke, D.O.; Hino, J.; Okano, I.; Kawano, Y.; Kohno, M.; Miyazato, M.; et al. Regulator of G-protein signaling subtype 4 mediates antihypertrophic effect of locally secreted natriuretic peptides in the heart. Circulation 2008, 117, 2329–2339. [Google Scholar] [CrossRef] [PubMed]
- Akabane, S.; Matsushima, Y.; Matsuo, H.; Kawamura, M.; Imanishi, M.; Omae, T. Effects of brain natriuretic peptide on renin secretion in normal and hypertonic saline-infused kidney. Eur. J. Pharmacol. 1991, 198, 143–148. [Google Scholar] [CrossRef]
- Kurtz, A.; Della Bruna, R.; Pfeilschifter, J.; Taugner, R.; Bauer, C. Atrial natriuretic peptide inhibits renin release from juxtaglomerular cells by a cGMP-mediated process. Proc. Natl. Acad. Sci. USA 1986, 83, 4769–4773. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Yoshimura, M.; Nakamura, S.; Nakayama, M.; Shimasaki, Y.; Harada, E.; Mizuno, Y.; Yamamuro, M.; Harada, M.; Saito, Y.; et al. Inhibitory effect of natriuretic peptides on aldosterone synthase gene expression in cultured neonatal rat cardiocytes. Circulation 2003, 107, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Kapoun, A.M.; Lam, A.; Damm, D.L.; Quan, D.; O’Connell, M.; Protter, A.A. B-Type natriuretic peptide inhibited angiotensin II-stimulated cholesterol biosynthesis, cholesterol transfer, and steroidogenesis in primary human adrenocortical cells. Endocrinology 2007, 148, 3722–3729. [Google Scholar] [CrossRef] [PubMed]
- Takekoshi, K.; Ishii, K.; Isobe, K.; Nomura, F.; Nammoku, T.; Nakai, T. Effects of natriuretic peptides (ANP, BNP, CNP) on catecholamine synthesis and TH mRNA levels in PC12 cells. Life Sci. 2000, 66, PL303–PL311. [Google Scholar] [CrossRef]
- Han, B.; Hasin, Y. Cardiovascular effects of natriuretic peptides and their interrelation with endothelin-1. Cardiovasc. Drugs Ther. 2003, 17, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.I.; Hodsman, P.G.; Kohzuki, M.; Casley, D.J.; Fabris, B.; Phillips, P.A. Interaction between atrial natriuretic peptide and the renin angiotensin aldosterone system. Endogenous antagonists. Am. J. Med. 1989, 87, 24S–28S. [Google Scholar] [PubMed]
- Rossi, F.; Mascolo, A.; Mollace, V. The pathophysiological role of natriuretic peptide-RAAS cross talk in heart failure. Int. J. Cardiol. 2017, 226, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Filippatos, G.; Farmakis, D.; Parissis, J.; Lekakis, J. Drug therapy for patients with systolic heart failure after the PARADIGM-HF trial: In need of a new paradigm of LCZ696 implementation in clinical practice. BMC Med. 2015, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Bayes-Genis, A.; Barallat, J.; Galan, A.; de Antonio, M.; Domingo, M.; Zamora, E.; Urrutia, A.; Lupon, J. Soluble neprilysin is predictive of cardiovascular death and heart failure hospitalization in heart failure patients. J. Am. Coll. Cardiol. 2015, 65, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Colucci, W.S.; Elkayam, U.; Horton, D.P.; Abraham, W.T.; Bourge, R.C.; Johnson, A.D.; Wagoner, L.E.; Givertz, M.M.; Liang, C.S.; Neibaur, M.; et al. Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure. Nesiritide Study Group. N. Engl. J. Med. 2000, 343, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Partovian, C.; Li, S.X.; Xu, X.; Lin, H.; Strait, K.M.; Hwa, J.; Krumholz, H.M. Patterns of change in nesiritide use in patients with heart failure: How hospitals react to new information. JACC Heart Fail. 2013, 1, 318–324. [Google Scholar] [CrossRef]
- Kostis, J.B.; Packer, M.; Black, H.R.; Schmieder, R.; Henry, D.; Levy, E. Omapatrilat and enalapril in patients with hypertension: The Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial. Am. J. Hypertens. 2004, 17, 103–111. [Google Scholar] [CrossRef] [PubMed]
- McCormack, P.L. Sacubitril/Valsartan: A Review in Chronic Heart Failure with Reduced Ejection Fraction. Drugs 2016, 76, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Volpe, M.; Rubattu, S.; Battistoni, A. ARNi: A Novel Approach to Counteract Cardiovascular Diseases. Int. J. Mol. Sci. 2019, 20, 2092. [Google Scholar] [CrossRef] [PubMed]
- Braunwald, E. The path to an angiotensin receptor antagonist-neprilysin inhibitor in the treatment of heart failure. J. Am. Coll. Cardiol. 2015, 65, 1029–1041. [Google Scholar] [CrossRef]
- Roksnoer, L.C.; van Veghel, R.; van Groningen, M.C.; de Vries, R.; Garrelds, I.M.; Bhaggoe, U.M.; van Gool, J.M.; Friesema, E.C.; Leijten, F.P.; Hoorn, E.J.; et al. Blood pressure-independent renoprotection in diabetic rats treated with AT1 receptor-neprilysin inhibition compared with AT1 receptor blockade alone. Clin. Sci. 2016, 130, 1209–1220. [Google Scholar] [CrossRef]
- Von Lueder, T.G.; Wang, B.H.; Kompa, A.R.; Huang, L.; Webb, R.; Jordaan, P.; Atar, D.; Krum, H. Angiotensin receptor neprilysin inhibitor LCZ696 attenuates cardiac remodeling and dysfunction after myocardial infarction by reducing cardiac fibrosis and hypertrophy. Circ. Heart Fail. 2015, 8, 71–78. [Google Scholar] [CrossRef]
- Haynes, R.; Judge, P.K.; Staplin, N.; Herrington, W.G.; Storey, B.C.; Bethel, A.; Bowman, L.; Brunskill, N.; Cockwell, P.; Hill, M.; et al. Effects of Sacubitril/Valsartan Versus Irbesartan in Patients With Chronic Kidney Disease. Circulation 2018, 138, 1505–1514. [Google Scholar] [CrossRef]
- Ruilope, L.M.; Dukat, A.; Bohm, M.; Lacourciere, Y.; Gong, J.; Lefkowitz, M.P. Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: A randomised, double-blind, placebo-controlled, active comparator study. Lancet 2010, 375, 1255–1266. [Google Scholar] [CrossRef]
- Solomon, S.D.; Zile, M.; Pieske, B.; Voors, A.; Shah, A.; Kraigher-Krainer, E.; Shi, V.; Bransford, T.; Takeuchi, M.; Gong, J.; et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: A phase 2 double-blind randomised controlled trial. Lancet 2012, 380, 1387–1395. [Google Scholar] [CrossRef]
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Vardeny, O.; Miller, R.; Solomon, S.D. Combined neprilysin and renin-angiotensin system inhibition for the treatment of heart failure. JACC Heart Fail. 2014, 2, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.S.; McMurray, J.J.; Packer, M.; Swedberg, K.; Rouleau, J.L.; Chen, F.; Gong, J.; Rizkala, A.R.; Brahimi, A.; Claggett, B.; et al. Effect of the angiotensin-receptor-neprilysin inhibitor LCZ696 compared with enalapril on mode of death in heart failure patients. Eur. Heart J. 2015, 36, 1990–1997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Packer, M.; McMurray, J.J.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation 2015, 131, 54–61. [Google Scholar] [CrossRef]
- Voors, A.A.; Gori, M.; Liu, L.C.; Claggett, B.; Zile, M.R.; Pieske, B.; McMurray, J.J.; Packer, M.; Shi, V.; Lefkowitz, M.P.; et al. Renal effects of the angiotensin receptor neprilysin inhibitor LCZ696 in patients with heart failure and preserved ejection fraction. Eur. J. Heart Fail. 2015, 17, 510–517. [Google Scholar] [CrossRef] [Green Version]
- Ishii, M.; Kaikita, K.; Sato, K.; Sueta, D.; Fujisue, K.; Arima, Y.; Oimatsu, Y.; Mitsuse, T.; Onoue, Y.; Araki, S.; et al. Cardioprotective Effects of LCZ696 (Sacubitril/Valsartan) After Experimental Acute Myocardial Infarction. JACC Basic Transl. Sci. 2017, 2, 655–668. [Google Scholar] [CrossRef]
- Campbell, D.J.; Krum, H.; Esler, M.D. Losartan increases bradykinin levels in hypertensive humans. Circulation 2005, 111, 315–320. [Google Scholar] [CrossRef]
- Grimm, M.O.; Mett, J.; Stahlmann, C.P.; Haupenthal, V.J.; Zimmer, V.C.; Hartmann, T. Neprilysin and Abeta Clearance: Impact of the APP Intracellular Domain in NEP Regulation and Implications in Alzheimer’s Disease. Front. Aging Neurosci. 2013, 5, 98. [Google Scholar] [CrossRef]
- Kuwahara, M.; Marumo, F. Biosynthesis of hormones in renal tubular and interstitial cells. Nihon Rinsho 1995, 53, 1873–1878. [Google Scholar] [PubMed]
- Marks, L.S.; Maxwell, M.H. Tigerstedt and the discovery of renin. An historical note. Hypertension 1979, 1, 384–388. [Google Scholar] [CrossRef] [PubMed]
- De Miguel, C.; Speed, J.S.; Kasztan, M.; Gohar, E.Y.; Pollock, D.M. Endothelin-1 and the kidney: New perspectives and recent findings. Curr. Opin. Nephrol. Hypertens. 2016, 25, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Boesen, E.I. Endothelin receptors, renal effects and blood pressure. Curr. Opin. Pharmacol. 2015, 21, 25–34. [Google Scholar] [CrossRef]
- Nasrallah, R.; Hassouneh, R.; Hebert, R.L. PGE2, Kidney Disease, and Cardiovascular Risk: Beyond Hypertension and Diabetes. J. Am. Soc. Nephrol. 2016, 27, 666–676. [Google Scholar] [CrossRef]
- Li, Y.; Xia, W.; Zhao, F.; Wen, Z.; Zhang, A.; Huang, S.; Jia, Z.; Zhang, Y. Prostaglandins in the pathogenesis of kidney diseases. Oncotarget 2018, 9, 26586–26602. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wei, Y.; Zheng, F.; Guan, Y.; Zhang, X. Prostaglandin E2 in the Regulation of Water Transport in Renal Collecting Ducts. Int. J. Mol. Sci. 2017, 18, 2539. [Google Scholar] [CrossRef]
- Tomita, K.; Endou, H.; Sakai, F. Localization of kallikrein-like activity along a single nephron in rabbits. Pflug. Arch. 1981, 389, 91–95. [Google Scholar] [CrossRef]
- Omata, K.; Carretero, O.A.; Scicli, A.G.; Jackson, B.A. Localization of active and inactive kallikrein (kininogenase activity) in the microdissected rabbit nephron. Kidney Int. 1982, 22, 602–607. [Google Scholar] [CrossRef] [Green Version]
- Proud, D.; Perkins, M.; Pierce, J.V.; Yates, K.N.; Highet, P.F.; Herring, P.L.; Mangkornkanok/Mark, M.; Bahu, R.; Carone, F.; Pisano, J.J. Characterization and localization of human renal kininogen. J. Biol. Chem. 1981, 256, 10634–10639. [Google Scholar]
- Rhaleb, N.E.; Yang, X.P.; Carretero, O.A. The kallikrein-kinin system as a regulator of cardiovascular and renal function. Compr. Physiol. 2011, 1, 971–993. [Google Scholar] [PubMed]
- Kauker, M.L. Bradykinin action on the efflux of luminal 22Na in the rat nephron. J. Pharmacol. Exp. Ther. 1980, 214, 119–123. [Google Scholar] [PubMed]
- Sinaiko, A.R.; Glasser, R.J.; Gillum, R.F.; Prineas, R.J. Urinary kallikrein excretion in grade school children with high and low blood pressure. J. Pediatr. 1982, 100, 938–940. [Google Scholar] [CrossRef]
- Sharma, J.N.; Narayanan, P. The kallikrein-kinin pathways in hypertension and diabetes. Prog. Drug Res. 2014, 69, 15–36. [Google Scholar] [PubMed]
- Wollheim, E.; Peterknecht, S.; Dees, C.; Wiener, A.; Wollheim, C.B. Defect in the excretion of a vasoactive polypeptide fraction A possible genetic marker of primary hypertension. Hypertension 1981, 3, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.J.; Woods, R.L.; Evans, R.G.; Alcorn, D.; Christy, I.J.; Anderson, W.P. Evidence for a renomedullary vasodepressor hormone. Clin. Exp. Pharmacol. Physiol. 1996, 23, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Muirhead, E.E. Renal vasodepressor mechanisms: The medullipin system. J. Hypertens. Suppl. 1993, 11, S53–S58. [Google Scholar] [CrossRef] [PubMed]
- Maric, C.; Harris, P.J.; Alcorn, D. Changes in mean arterial pressure predict degranulation of renomedullary interstitial cells. Clin. Exp. Pharmacol. Physiol. 2002, 29, 1055–1059. [Google Scholar] [CrossRef]
- Glodny, B.; Pauli, G.F. The vasodepressor function of the kidney: Prostaglandin E2 is not the principal vasodepressor lipid of the renal medulla. Acta Physiol. 2006, 187, 419–430. [Google Scholar] [CrossRef]
- Pitcock, J.A.; Brown, P.S.; Byers, W.; Brooks, B.; Muirhead, E.E. Degranulation of renomedullary interstitial cells during reversal of hypertension. Hypertension 1981, 3, II-75. [Google Scholar] [CrossRef]
- Wang, T.J.; Larson, M.G.; Levy, D.; Leip, E.P.; Benjamin, E.J.; Wilson, P.W.; Sutherland, P.; Omland, T.; Vasan, R.S. Impact of age and sex on plasma natriuretic peptide levels in healthy adults. Am. J. Cardiol. 2002, 90, 254–258. [Google Scholar] [CrossRef]
- Redfield, M.M.; Rodeheffer, R.J.; Jacobsen, S.J.; Mahoney, D.W.; Bailey, K.R.; Burnett, J.C., Jr. Plasma brain natriuretic peptide concentration: Impact of age and gender. J. Am. Coll. Cardiol. 2002, 40, 976–982. [Google Scholar] [CrossRef]
- Sayama, H.; Nakamura, Y.; Saito, N.; Kinoshita, M. Why is the concentration of plasma brain natriuretic peptide in elderly inpatients greater than normal? Coron. Artery Dis. 1999, 10, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, K.; Nieves Castro, D.K.; Fu, Q.; Gottlieb, R.A.; Van Eyk, J.E.; Merz, C.N.B. Sex differences in ischemic heart disease and heart failure biomarkers. Biol. Sex Differ. 2018, 9, 43. [Google Scholar] [CrossRef] [PubMed]



 English
English Bahasa Melayu
Bahasa Melayu Bahasa Indonesia
Bahasa Indonesia Tiếng Việt
Tiếng Việt ไทย
ไทย