中文品名:羅氏可霸斯心肌酵素定量儀 (未滅菌) 英文品名: "Roche" cobas h 232 (Non-sterile) 醫材字號:衛署醫器輸壹字第005621號 效能:限醫療器材分類分級管理辦法「臨床使用微量化學分析儀(A.2170)」第一等級鑑別範圍。 申請商名稱:台灣羅氏醫療診斷設備股份有限公司 申請商地址:台北市中山區民權東路3段2號10樓 製造廠名稱:ROCHE DIAGNOSTICS GMBH 製造廠廠址:SANDHOFER STRASSE 116 D-68305 MANNHEIM GERMANY
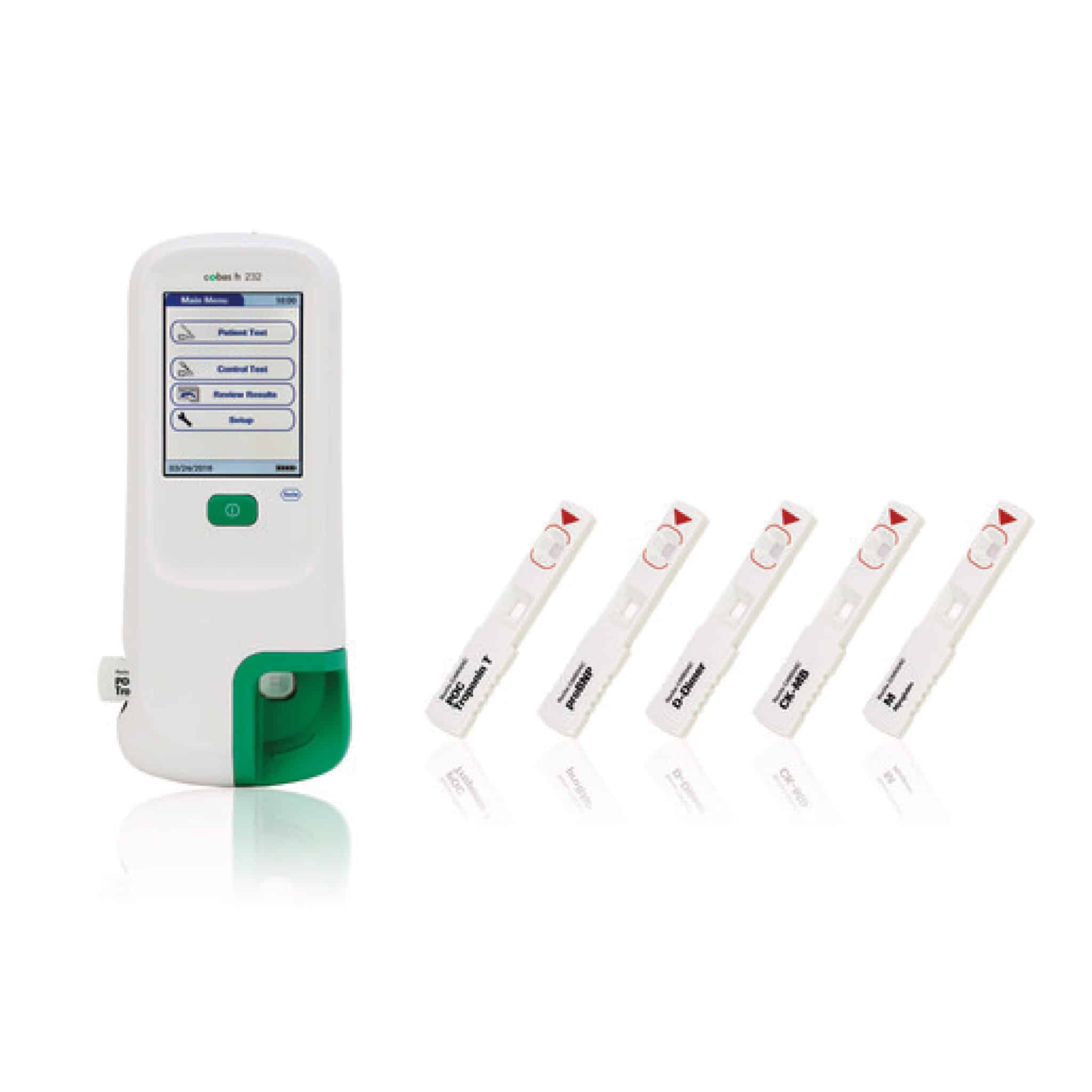
cobas® h 232 POC system
Aiding confident on-the-spot diagnosis and management of patients presenting with signs and symptoms of cardiovascular disease
On-the-spot care & share
For Frontline Healthcare Providers, cobas h 232 POC system is a portable point-of-care system that supports optimized treatment of patients with symptoms of chest pain and dyspnea, because it enables confident on-the-spot diagnosis and assessment of the patient’s condition based on objective results, based on objective results, that can be compared with the Roche laboratory methods and shared wirelessly for immediate feedback and response.
Benefits
The ideal fit for “on-the-spot care and share” in pre-hospital settings and emergency rooms settings
Fast
On-the-spot results are available in 3 steps and 12 minutes or less1,2,3,4,5
Portable
Handheld point of care system is lightweight and easy to use, even in mobile situation6
Connected
Wireless technology ensures immediate availability of results at all Points of Care (requires cobas® infinity POC solution)6
Confident
Accurate results, aligned with Roche central laboratory tests7
Safety
Operator ID entry and lockout to ensure use by authorized staff
Patient and user ID to ensure correct documentation of test results
Quality control lockout
Control and traceability
。Enhanced connectivity through wireless technology and a unique QR code feature can minimize errors, increase safety and streamline workflow
。Connection to the cobas® infinity POC solution allows extension of the testing network and ensures control of operators and quality assurance from the central laboratory
。Automatic recertification of operators through cobas academy to ensure use by trained operators only
Available tests
The cobas h 232 POC system allows rapid and easy determination of Troponin T, NT-proBNP, D-Dimer, CK-MB and Myoglobin in different settings, like ambulances, general practitioner offices and emergency rooms.
Test |
Measuring range |
Time to result |
Clinical utility |
Troponin |
40 – 2,000 ng/L | 12 min | Early aid in diagnosis of acute myocardial infarction and identification of patients with an elevated mortality risk4 |
NT-proBNP |
60 – 9,000 pg/mL | 12 min | Aid in diagnosis of patients with suspected heart failure, in monitoring of patients with compensated left ventricular dysfunction and in the risk stratification of patients with acute coronary syndromes5 |
CK-MB |
1.0 – 40 ng/mL | 12 min | Aid in diagnosis of patients with suspected acute myocardial infarction (AMI, heart attack), assessment of the size of the infarction and detection of re-infarction1 |
D-Dimer |
0.1 – 4.0 µg/mL | 8 min | Aid in exclusion of deep venous thrombosis and pulmonary embolism2 |
Myoglobin |
30 – 700 ng/mL | 8 min | Aid in diagnosis of patients with suspected myocardial infarction, reperfusion control3 |
Faster triaging of patients with suspected acute myocardial infarction in pre-hospital care and emergency room4,8
A test result from Roche CARDIAC POC Troponin T ≥ 50ng/L allows identification of patients at high risk of mortality and helps ensure a fast triage to coronary intensive care unit or cath lab.
How the cobas h 232 POC system works
Rapid and easy determination of cardiac biomarkers
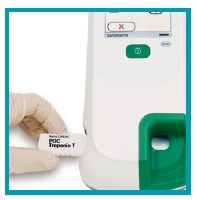 |
| 1. Insert test strip |
 |
| 2. Apply sample of 150 μL heparinized whole blood using the Roche cardiac pipette
8–12 minutes |
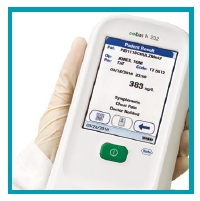 |
| 3. Read result |
References
1 Bertsch, T. et al. (2010). Clin Lab. 56(1-2), 37-49.
2 Roche (2016). cobas h 232 POC system Operator’s Manual, Version 6.0.
3 Roche CARDIAC D-Dimer-Method Sheet-package insert.
4 Roche CARDIAC proBNP-Method Sheet-package insert.
5 Roche CARDIAC POC Troponin T-Method Sheet-package insert.
6 Roche CARDIAC CK-MB-Method Sheet-package insert.
7 Roche CARDIAC M-Method Sheet-package insert.
8 Konstantinides, S. et al. (2014). Eur Heart J 35, 3033-3080.
9 Ponikowski, P. et al. (2016). Eur J Heart Fail 18(8), 891-975.
10 Roffi , M. et al. (2015). Eur Heart J 37(3), 267-315.
11 Stengaard, C. et al. (2013). American J Cardiol 112(9), 1361-1366.
12 Achar, S.A. et al. (2005). Am Fam Physician 72(1), 119-126.
13 Jungbauer, C. et al. (2017). Clin Lab 63(4), 633-645.
14 De Bastos, M.M. et al. (2008). Blood Coagul Fibrinolysis 19(1),
48–54.
15 Wells, P.S. et al. (2003). N Engl J Med 349(13), 416-420.
16 Berliner, D. et al. (2016). Dtsch Arztebl Int 113(49), 834-845.
17 Taylor, C.J. et al. (2017). Br J Gen Pract. 67(655), e94-e102.
18 Taylor, C.J. et al. (2017). Effi cacy and Mechanism Evaluation, No. 4.3. National Institute for Health. Research. ISSN 2050-4365. [Accessed September 2018].
19 British Heart Foundation and the All-Party Parliamentary Group on Heart Disease (2016). Focus on Heart Failure. Report accessible on https://www.bhf.org.uk/get-involved/campaigning/inquiry-
intoliving-with-heart-failure [Accessed September 2018].
20 Januzzi, J.L. et al. (2006). Eur Heart J 27(3), 330-337.
21 Januzzi, J.L. et al. (2018). J Am Coll Cardiol 71(11), 1191-1200.
22 Masson, S. et al. (2008). J Am Coll Cardiol 52, 997-1000.
23 DeBeradinis, B., Januzzi, J.L. (2012). Curr Opin Cardiol 27(6):
661-668.
24 Chiong, J. (2010). Heart Fail Rev. 15(4), 275-291.
25 Weiner, R. (2012). Eur J Heart Fail 15(3), 342-351.
26 Januzzi (2012). Arch Cardiovasc Dis. 105(1), 40-50.
27 Januzzi, J.L. et al. (2016). Clin Chem 62(5), 663-665.
28 Stengaard, C. et al. (2016). European Heart Journal: Acute Cardiovascular Care, 1-10.



 English
English Bahasa Melayu
Bahasa Melayu Bahasa Indonesia
Bahasa Indonesia Tiếng Việt
Tiếng Việt ไทย
ไทย